Acute Coronary Syndrome
- 14 feb 2020
- 87 Min. de lectura
Practice Essentials
Acute coronary syndrome (ACS) refers to a spectrum of clinical presentations ranging from those for ST-segment elevation myocardial infarction (STEMI) to presentations found in non–ST-segment elevation myocardial infarction (NSTEMI) or in unstable angina. It is almost always associated with rupture of an atherosclerotic plaque and partial or complete thrombosis of the infarct-related artery.

Suggested algorithm for triaging patients with chest pain. ACS = ACS; ASA = aspirin; EKG = ECG; MI = myocardial infarction; Rx = treat; STEMI = ST-elevation myocardial infarction. Courtesy of Wu et al (1999).
See Are You Missing Subtle MI Clues on ECGs? Test Your Skills, a Critical Images slideshow, to help identify a variety of electrocardiographic abnormalities.
Signs and symptoms
Atherosclerosis is the primary cause of ACS, with most cases occurring from the disruption of a previously nonsevere lesion. Complaints reported by patients with ACS include the following:
Palpitations
Pain, which is usually described as pressure, squeezing, or a burning sensation across the precordium and may radiate to the neck, shoulder, jaw, back, upper abdomen, or either arm
Exertional dyspnea that resolves with pain or rest
Diaphoresis from sympathetic discharge
Nausea from vagal stimulation
Decreased exercise tolerance
Physical findings can range from normal to any of the following:
Hypotension: Indicates ventricular dysfunction due to myocardial ischemia, myocardial infarction (MI), or acute valvular dysfunction
Hypertension: May precipitate angina or reflect elevated catecholamine levels due to anxiety or to exogenous sympathomimetic stimulation
Diaphoresis
Pulmonary edema and other signs of left heart failure
Extracardiac vascular disease
Jugular venous distention
Cool, clammy skin and diaphoresis in patients with cardiogenic shock
A third heart sound (S3) and, frequently, a fourth heart sound (S4)
A systolic murmur related to dynamic obstruction of the left ventricular outflow tract
Rales on pulmonary examination (suggestive of left ventricular dysfunction or mitral regurgitation)
Potential complications include the following:
Ischemia: Pulmonary edema
Myocardial infarction: Rupture of the papillary muscle, left ventricular free wall, and ventricular septum
See Presentation for more detail.
Diagnosis
Guidelines for the management of non-ST-segment elevation ACS were released in 2011 by the European Society of Cardiology (ESC). [1] The guidelines include the use of the CRUSADE risk score (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines).
In the emergency setting, electrocardiography (ECG) is the most important diagnostic test for angina. ECG changes that may be seen during anginal episodes include the following:
Transient ST-segment elevations
Dynamic T-wave changes: Inversions, normalizations, or hyperacute changes
ST depressions: These may be junctional, downsloping, or horizontal
Laboratory studies that may be helpful include the following:
Creatine kinase isoenzyme MB (CK-MB) levels
Cardiac troponin levels
Myoglobin levels
Complete blood count
Basic metabolic panel
Diagnostic imaging modalities that may be useful include the following:
Chest radiography
Echocardiography
Myocardial perfusion imaging
Cardiac angiography
Computed tomography, including CT coronary angiography and CT coronary artery calcium scoring
See Workup for more detail.
Management
Initial therapy focuses on the following:
Stabilizing the patient’s condition
Relieving ischemic pain
Providing antithrombotic therapy
Pharmacologic anti-ischemic therapy includes the following:
Nitrates (for symptomatic relief)
Beta blockers (eg, metoprolol): These are indicated in all patients unless contraindicated
Pharmacologic antithrombotic therapy includes the following:
Aspirin
Clopidogrel
Prasugrel
Ticagrelor
Glycoprotein IIb/IIIa receptor antagonists (abciximab, eptifibatide, tirofiban)
Pharmacologic anticoagulant therapy includes the following:
Unfractionated heparin (UFH)
Low-molecular-weight heparin (LMWH; dalteparin, nadroparin, enoxaparin)
Factor Xa inhibitors (rivaroxaban, fondaparinux)
Additional therapeutic measures that may be indicated include the following:
Thrombolysis
Percutaneous coronary intervention (preferred treatment for ST-elevation MI)
Current guidelines for patients with moderate- or high-risk ACS include the following:
Early invasive approach
Concomitant antithrombotic therapy, including aspirin and clopidogrel, as well as UFH or LMWH
See Treatment and Medication for more detail.
The image below depicts a 62-year-old woman with a history of chronic stable angina and a "valve problem."

A 62-year-old woman with a history of chronic stable angina and a "valve problem" presents with new chest pain. She is symptomatic on arrival, complaining of shortness of breath and precordial chest tightness. Her initial vital signs are blood pressure = 140/90 mm Hg and heart rate = 98. Her electrocardiogram (ECG) is as shown. She is given nitroglycerin sublingually, and her pressure decreases to 80/palpation. Right ventricular ischemia should be considered in this patient.View Media Gallery
Background
Acute coronary syndrome (ACS) refers to a spectrum of clinical presentations ranging from those for ST-segment elevation myocardial infarction (STEMI) to presentations found in non–ST-segment elevation myocardial infarction (NSTEMI) or in unstable angina. In terms of pathology, ACS is almost always associated with rupture of an atherosclerotic plaque and partial or complete thrombosis of the infarct-related artery. (See Etiology.)
In some instances, however, stable coronary artery disease (CAD) may result in ACS in the absence of plaque rupture and thrombosis, when physiologic stress (eg, trauma, blood loss, anemia, infection, tachyarrhythmia) increases demands on the heart. The diagnosis of acute myocardial infarction in this setting requires a finding of the typical rise and fall of biochemical markers of myocardial necrosis in addition to at least 1 of the following [2] (See Workup.):
Ischemic symptoms
Development of pathologic Q waves on electrocardiogram (ECG)
Significant ST-segment-T wave (ST-T) changes or new left bundle branch block (LBBB)
Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
Introcoronary thrombus identified by angiography or autopsy
The terms transmural and nontransmural (subendocardial) myocardial infarction are no longer used because ECG findings in patients with this condition are not closely correlated with pathologic changes in the myocardium. Therefore, a transmural infarct may occur in the absence of Q waves on ECGs, and many Q-wave myocardial infarctions may be subendocardial, as noted on pathologic examination. Because elevation of the ST segment during ACS is correlated with coronary occlusion and because it affects the choice of therapy (urgent reperfusion therapy), ACS-related myocardial infarction should be designated STEMI or NSTEMI. (See Workup.)
Attention to the underlying mechanisms of ischemia is important when managing ACS. A simple predictor of demand is rate-pressure product, which can be lowered by beta blockers (eg, metoprolol or atenolol) and pain/stress relievers (eg, morphine), while supply may be improved by oxygen, adequate hematocrit, blood thinners (eg, heparin, IIb/IIIa agents such as abciximab, eptifibatide, tirofiban, or thrombolytics), and/or vasodilators (eg, nitrates, amlodipine). (See Medication.)
Etiology
Acute coronary syndrome (ACS) is caused primarily by atherosclerosis. Most cases of ACS occur from disruption of a previously nonsevere lesion (an atherosclerotic lesion that was previously hemodynamically insignificant yet vulnerable to rupture). The vulnerable plaque is typified by a large lipid pool, numerous inflammatory cells, and a thin, fibrous cap.
Elevated demand can produce ACS in the presence of a high-grade fixed coronary obstruction, due to increased myocardial oxygen and nutrition requirements, such as those resulting from exertion, emotional stress, or physiologic stress (eg, from dehydration, blood loss, hypotension, infection, thyrotoxicosis, or surgery).
ACS without elevation in demand requires a new impairment in supply, typically due to thrombosis and/or plaque hemorrhage.
The major trigger for coronary thrombosis is considered to be plaque rupture caused by the dissolution of the fibrous cap, the dissolution itself being the result of the release of metalloproteinases (collagenases) from activated inflammatory cells. This event is followed by platelet activation and aggregation, activation of the coagulation pathway, and vasoconstriction. This process culminates in coronary intraluminal thrombosis and variable degrees of vascular occlusion. Distal embolization may occur. The severity and duration of coronary arterial obstruction, the volume of myocardium affected, the level of demand on the heart, and the ability of the rest of the heart to compensate are major determinants of a patient's clinical presentation and outcome. (Anemia and hypoxemia can precipitate myocardial ischemia in the absence of severe reduction in coronary artery blood flow.)
A syndrome consisting of chest pain, ischemic ST-segment and T-wave changes, elevated levels of biomarkers of myocyte injury, and transient left ventricular apical ballooning (takotsubo syndrome) has been shown to occur in the absence of clinical CAD, after emotional or physical stress. The etiology of this syndrome is not well understood but is thought to relate to a surge of catechol stress hormones and/or high sensitivity to those hormones.
Baseline blood glucose levels appear to be an independent risk factor for a major adverse cardiac event (MACE) in emergency department (ED) patients with suspected ACS. [3, 4] In an analysis of data from 1708 Australian and New Zealand patients in a prospective observational study, investigators noted a MACE occurred within 30 days of presentation in 15.3% of patients whose ED admission blood glucose levels were below 7 mmol/L (about 126 mg/dL); however, in the same time period, a MACE occurred in twice as many patients (30.9%) whose blood glucose levels were above 7 mmol/L. [4] After controlling for various factors, patients who had admission blood glucose levels of 7 mmol/L or higher were at 51% higher risk of experiencing a MACE compared with patients who had lower baseline blood glucose levels. [4] Other significant predictors of MACE included male sex, older age, family history, hypertension, dyslipidemia, ischemic findings on ECG, and positive troponintests. [3, 4]
Prognosis
Six-month mortality rates in the Global Registry of Acute Coronary Events (GRACE) were 13% for patients with NSTEMI ACS and 8% for those with unstable angina.
An elevated level of troponin (a type of regulatory protein found in skeletal and cardiac muscle) permits risk stratification of patients with ACS and identifies patients at high risk for adverse cardiac events (ie, myocardial infarction, death) up to 6 months after the index event. [5, 6] (See Workup.)
The PROVE IT-TIMI trial found that after ACS, a J-shaped or U-shaped curve association is observed between BP and the risk of future cardiovascular events. [7]
LeLeiko et al determined that serum choline and free F(2)-isoprostane are also predictors of cardiac events in ACS. The authors evaluated the prognostic value of vascular inflammation and oxidative stress biomarkers in patients with ACS to determine their role in predicting 30-day clinical outcomes. Serum F(2)-isoprostane had an optimal cutoff level of 124.5 pg/mL, and serum choline had a cutoff level of 30.5 µmol/L. Choline and F(2)-isoprostane had a positive predictive value of 44% and 57% and a negative predictive value of 89% and 90%, respectively. [8]
Testosterone deficiency is common in patients with coronary disease and has a significant negative impact on mortality. Further study is needed to assess the effect of treatment on survival. [9]
A study by Sanchis et al suggests renal dysfunction, dementia, peripheral artery disease, previous heart failure, and previous myocardial infarction are the comorbid conditions that predict mortality in NSTEMI ACS. [10] In patients with comorbid conditions, the highest risk period was in the first weeks after NSTEMI ACS. In-hospital management of patients with comorbid conditions merits further investigation.
Patients with end-stage renal disease often develop ACS, and little is known about the natural history of ACS in patients receiving dialysis. Gurm et al examined the presentation, management, and outcomes of patients with ACS who received dialysis before presentation for an ACS. These patients were enrolled in the Global Registry of Acute Coronary Events (GRACE) at 123 hospitals in 14 countries from 1999-2007.
NSTEMI ACS was the most common in patients receiving dialysis, occurring in 50% of patients (290 of 579) versus 33% (17,955 of 54,610) of those not receiving dialysis The in-hospital mortality rates were higher among patients receiving dialysis (12% vs 4.8%; p < 0.0001). Higher 6-month mortality rates (13% vs 4.2%; p < 0.0001), recurrent myocardial infarction incidence (7.6% vs 2.9%; p < 0.0001), and unplanned rehospitalizations (31% vs 18%; p < 0.0001) were found among those who survived to discharge. Outcomes in patients who received dialysis was worse than was predicted by the calculated GRACE risk score for in-hospital mortality (7.8% predicted vs 12% observed; p < 0.05). This suggests that the GRACE risk score underestimated the risk of major events in these patients. [11]
In a study that assessed the impact of prehospital time on STEMI outcome, Chughatai et al suggest that "total time to treatment" should be used as a core measure instead of "door-to-balloon time." [12] This is because on-scene time was the biggest fraction of "pre-hospital time." The study compared groups with total time to treatment of more than 120 minutes compared with 120 minutes or less and found mortalities were 4 compared with 0 and transfers to a tertiary care facility were 3 compared with 1, respectively.
STEMI mechanisms and stenting outcome similar in women and men
Despite their smaller coronary vessels and higher risk profile, women with STEMI appear to respond just as well as men to primary PCI and stenting, according to the Optical Coherence Tomography Assessment of Gender Diversity in Primary Angioplasty (OCTAVIA) study. [13] OCTAVIA, which was designed to examine gender differences at the time of primary PCI, included 140 STEMI patients at 14 Italian centers, matched by age and risk factors, who received an everolimus-eluting stent. [13]
On initial OCT, no differences by gender were found in the proportion of ruptured or eroded plaques, thus suggesting that the pathophysiology of STEMI is nearly identical in men and women. [13] On repeat OCT at nine months, intended to assess stent healing, more than 90% of both men and women had fully covered stent struts. Although OCTAVIA was not powered for clinical end points, no significant differences in death, reinfarction, stroke, stent thrombosis, or target vessel reintervention were evident at one year. [13]
Complications
Complications of ischemia include pulmonary edema, whereas those of myocardial infarction include rupture of the papillary muscle, left ventricular free wall, and ventricular septum.
Patient Education
Patient education of risk factors is important, but more attention is needed regarding delays in door-to-balloon time, and one major barrier to improving this delay is patient education regarding his or her symptoms. Lack of recognition of symptoms may cause tremendous delays in seeking medical attention.
Educate patients about the dangers of cigarette smoking, a major risk factor for coronary artery disease (CAD). The risk of recurrent coronary events decreases 50% at 1 year after smoking cessation. Provide all patients who smoke with guidance, education, and support to avoid smoking. Smoking-cessation classes should be offered to help patients avoid smoking after a myocardial infarction. Bupropion increases the likelihood of successful smoking cessation.
Diet plays an important role in the development of CAD. Therefore, prior to hospital discharge, a patient who has had a myocardial infarction should be evaluated by a dietitian. Patients should be informed about the benefits of a low-cholesterol, low-salt diet. In addition, educate patients about AHA dietary guidelines regarding a low-fat, low-cholesterol diet.
A cardiac rehabilitation program after discharge may reinforce education and enhance compliance.
The following mnemonic may useful in educating patients with CAD regarding treatments and lifestyle changes necessitated by their condition:
A = Aspirin and antianginals
B = Beta blockers and blood pressure (BP)
C = Cholesterol and cigarettes
D = Diet and diabetes
E = Exercise and education
For patients being discharged home, emphasize the following:
Timely follow-up with primary care provider
Compliance with discharge medications, specifically aspirin and other medications used to control symptoms
Need to return to the ED for any change in frequency or severity of symptoms
For patient education resources, see the Heart Health Center and Cholesterol Center, as well as High Cholesterol, Cholesterol Charts (What the Numbers Mean), Lifestyle Cholesterol Management, Chest Pain, Coronary Heart Disease, Heart Attack, Angina Pectoris, Cholesterol-Lowering Medications, and Statins (Cholesterol Drugs).
Acute Coronary Syndrome Clinical Presentation
History
The severity and duration of coronary artery obstruction, the volume of myocardium affected, the level of demand, and the ability of the rest of the heart to compensate are major determinants of a patient's clinical presentation and outcome. A patient may present to the ED because of a change in pattern or severity of symptoms.
Typically, angina is a symptom of myocardial ischemia that appears in circumstances of increased oxygen demand. It is usually described as a sensation of chest pressure or heaviness that is reproduced by activities or conditions that increase myocardial oxygen demand. A new case of angina is more difficult to diagnose because symptoms are often vague and similar to those caused by other conditions (eg, indigestion, anxiety).
However, not all patients experience chest pain. They may present with only neck, jaw, ear, arm, or epigastric discomfort. Some patients, including some who are elderly or who have diabetes, present with no pain, complaining only of episodic shortness of breath, severe weakness, light-headedness, diaphoresis, or nausea and vomiting. Elderly persons may also present only with altered mental status. Those with preexisting altered mental status or dementia may have no recollection of recent symptoms and may have no complaints.
In addition, evidence exists that women more often have coronary events without typical symptoms, which may explain the frequent failure of clinicians to initially diagnose ACS in women.
A summary of patient complaints is as follows:
Palpitations
Pain, which is usually described as pressure, squeezing, or a burning sensation across the precordium and may radiate to the neck, shoulder, jaw, back, upper abdomen, or either arm
Exertional dyspnea that resolves with pain or rest
Diaphoresis from sympathetic discharge
Nausea from vagal stimulation
Decreased exercise tolerance
Stable angina involves episodic pain lasting 5-15 minutes, is provoked by exertion, and is relieved by rest or nitroglycerin. In unstable angina, patients have increased risk for adverse cardiac events, such as myocardial infarction or death. New-onset exertional angina can occur at rest and is of increasing frequency or duration or is refractory to nitroglycerin. Variant angina (Prinzmetal angina) occurs primarily at rest, is triggered by smoking, and is thought to be due to coronary vasospasm.
Physical Examination
Physical examination results are frequently normal. If chest pain is ongoing, the patient will usually lie quietly in bed and may appear anxious, diaphoretic, and pale. Physical findings can vary from normal to any of the following:
Hypotension - Indicates ventricular dysfunction due to myocardial ischemia, infarction, or acute valvular dysfunction
Hypertension - May precipitate angina or reflect elevated catecholamine levels due to anxiety or to exogenous sympathomimetic stimulation
Diaphoresis
Pulmonary edema and other signs of left heart failure
Extracardiac vascular disease
Jugular venous distention
Cool, clammy skin and diaphoresis in patients with cardiogenic shock
In addition, a third heart sound (S3) may be present, and frequently, a fourth heart sound (S4) exists. The latter is especially prevalent in patients with inferior-wall ischemia and may be heard in patients with ischemia or systolic murmur secondary to mitral regurgitation
A systolic murmur related to dynamic obstruction of the left ventricular (LV) outflow tract may also occur. It is caused by hyperdynamic motion of the basal left ventricular myocardium and may be heard in patients with an apical infarct.
A new murmur may reflect papillary muscle dysfunction. Rales on pulmonary examination may suggest LV dysfunction or mitral regurgitation.
Patients who present to the ED with chest pain who have a low short-term risk of a major adverse cardiac event must be identified to facilitate early discharge in order to avoid lengthy and costly hospital stays. [14] The ASPECT study tested a 2-hour, accelerated diagnostic protocol (ADP) that included the use of a structured pretest probability scoring method, electrocardiography, and a point-of-care biomarker panel that included troponin, creatine kinase MB, and myoglobin levels. The study suggests that ADP can identify patients at low risk for a short-term major adverse cardiac event who may be suitable for early discharge; such an approach could be used to decrease the overall observation periods and admissions for chest pain and has the potential to affect health-service delivery worldwide.
ACP Screening Guidelines for CHD
In 2015, the American College of Physicians (ACP) released guidelines on screening for coronary heart disease (CHD), including the following [15] :
There is no evidence that cardiac screening improves patient outcomes in asymptomatic, low-risk adults.
Potential harms of cardiac screening include false-positive results causing patients to undergo potentially unnecessary tests and procedures.
Among adults at low risk, prevalence of coronary heart disease is low, and cardiac screening is of low predictive value. Therefore, cardiac screening is of low yield, and the probability that positive findings will influence therapeutic decision making is low.
Clinicians should therefore emphasize strategies to reduce cardiovascular risk even further among low-risk adults by treating modifiable risk factors (smoking, diabetes, blood pressure, hyperlipidemia, overweight, and exercise).
Clinicians should not screen asymptomatic, low-risk adults for cardiac disease using resting or stress electrocardiography, stress echocardiography, or stress myocardial perfusion imaging.
Clinicians should conduct cardiovascular risk assessment with a global risk score combining individual risk factor measurements into a single quantitative estimate of risk.
The ACP recommendations do not apply to symptomatic patients or to screening athletes before participation in various events.
Diagnostic Considerations
As many as half of all cases of ACS are clinically silent in that they do not cause the classic symptoms of this syndrome. Consequently, ACS goes unrecognized by the patient. Maintain a high index of suspicion for ACS, especially when evaluating women, patients with diabetes, older patients, patients with dementia, and those with a history of heart failure.
Although ST-segment and T-wave changes are associated with CAD, alternative causes of these findings are left ventricular aneurysm, pericarditis, Prinzmetal angina, early repolarization, Wolff-Parkinson-White syndrome, and drug therapy (eg, with tricyclic antidepressants, phenothiazines).
Increasing public awareness of the typical and atypical presentations of ACS is of the utmost importance for optimal and timely treatment. Many patients do not recognize that their symptoms are cardiac in origin and therefore may delay seeking medical help. Patients with established CAD call emergency medical services if they have chest pain that does not resolve after they take a sublingual nitroglycerin tablet.
In patients presenting to the ED with chest pain, a structured diagnostic approach that includes time-focused ED decision points, brief observation, and selective application of early outpatient provocative testing appeared both safe and diagnostically efficient in a study by Scheuermeyer et al. However, some patients with ACS may be discharged for outpatient stress testing on the index ED visit. [16]
Differential Diagnoses
Hypertensive Emergencies in Emergency Medicine
Acute Coronary Syndrome Workup
Approach Considerations
Stable coronary artery disease (CAD) may result in ACS in the absence of plaque rupture and thrombosis, when physiologic stress (eg, trauma, blood loss, anemia, infection, tachyarrhythmias) increases demands on the heart. In such cases, the diagnosis of acute myocardial infarction can be made if workup reveals the typical rise and fall of biochemical markers of myocardial necrosis along with at least one of the following [2] :
Ischemic symptoms
Development of pathologic Q waves on electrocardiogram (ECG)
Significant ST-segment-T wave (ST-T) changes or new left bundle branch block (LBBB)
Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
Introcoronary thrombus identified by angiography or autopsy
Non–ST-segment elevation myocardial infarction (NSTEMI) is distinguished from unstable angina by elevated levels of cardiac enzymes and biomarkers of myocyte necrosis. Differentiation is generally based on 3 sets of biomarkers measured at 6- to 8-hour intervals after the patient's presentation to the ED. The current definition of NSTEMI requires a typical clinical syndrome plus elevated troponin (or creatine kinase isoenzyme MB [CK-MB]) levels to over 99% of the normal reference (with a coefficient of variation of < 10% for the assay). Given this definition, nearly 25% of patients who were previously classified as having unstable angina now fulfill the criteria for NSTEMI.
Measure cardiac enzyme levels at regular intervals, starting at admission and continuing until the peak is reached or until 3 sets of results are negative. Biochemical biomarkers (demonstrated in the image below) are useful for diagnosis and prognostication.

This plot shows changes in cardiac markers over time after the onset of symptoms. Peak A is the early release of myoglobin or creatine kinase isoenzyme MB (CK-MB) after acute myocardial infarction (AMI). Peak B is the cardiac troponin level after infarction. Peak C is the CK-MB level after infarction. Peak D is the cardiac troponin level after unstable angina. Data are plotted on a relative scale, where 1.0 is set at the myocardial-infarction cutoff concentration. Courtesy of Wu et al (1999). ROC = receiver operating characteristic.
Of note, cardiac-specific troponins are not detectable in the blood of healthy individuals; therefore, they provide high specificity for detecting injury to cardiac myocytes. These molecules are also more sensitive than CK-MB for myocardial necrosis and therefore improve early detection of small myocardial infarctions. Although blood troponin levels increase simultaneously with CK-MB levels (about 6 h after the onset of infarction), they remain elevated for as long as 2 weeks. As a result, troponin values cannot be used to diagnose reinfarction. New methods of detecting troponins in the blood can measure levels as low as 0.1-0.2 ng/mL.
Keller et al suggest that among patients with suspected acute coronary syndrome, highly sensitive troponin I assay (hsTnI) or contemporary troponin I assay (cTnI) determination 3 hours after admission for chest pain may facilitate early rule-out of acute myocardial infarction. A serial change in hsTnI or cTnI levels from admission (using the 99th percentile diagnostic cutoff value) to 3 hours postadmission may aid in early diagnosis of acute myocardial infarction. [17]
Minor elevations in these molecules can be detected in the blood of patients without ACS in the setting of myocarditis (pericarditis), sepsis, renal failure, acute congestive heart failure (CHF), acute pulmonary embolism, or prolonged tachyarrhythmias.
Electrocardiography
ECGs should be reviewed promptly. Involve a cardiologist when in doubt.
Recording an ECG during an episode of the presenting symptoms is valuable. Transient ST-segment changes (>0.05 mV) that develop during a symptomatic period and that resolve when the symptoms do are strongly predictive of underlying CAD and have prognostic value. Comparison with previous ECGs is often helpful.
Alternative causes of ST-segment and T-wave changes are left ventricular aneurysm, pericarditis, Prinzmetal angina, early repolarization, Wolff-Parkinson-White syndrome, and drug therapy (eg, with tricyclic antidepressants, phenothiazines).
In the emergency setting, ECG is the most important ED diagnostic test for angina. It may show changes during symptoms and in response to treatment, confirm a cardiac basis for symptoms. It also may demonstrate preexisting structural or ischemic heart disease (left ventricular hypertrophy, Q waves). A normal ECG or one that remains unchanged from the baseline does not exclude the possibility that chest pain is ischemic in origin. Changes that may be seen during anginal episodes include the following:
Transient ST-segment elevations
Dynamic T-wave changes - Inversions, normalizations, or hyperacute changes
ST depressions - May be junctional, downsloping, or horizontal
In patients with transient ST-segment elevations, consider LV aneurysm, pericarditis, Prinzmetal angina, early repolarization, and Wolff-Parkinson-White syndrome as possible diagnoses. Fixed changes suggest acute myocardial infarction.
When deep T-wave inversions are present, consider the possibility of central nervous system (CNS) events or drug therapy with tricyclic antidepressants or phenothiazines as the cause.
Diagnostic sensitivity may be increased by performing right-sided leads (V4 R), posterior leads (V8, V9), and serial recordings.
ECGs from two patients are shown below.

A 50-year-old man with type 1 diabetes mellitus and hypertension presents after experiencing 1 hour of midsternal chest pain that began after eating a large meal. Pain is now present but is minimal. Aspirin is the single drug that will have the greatest potential impact on subsequent morbidity. In the setting of ongoing symptoms and electrocardiogram (ECG) changes, nitrates titrated to 10% reduction in blood pressure and symptoms, beta blockers, and heparin are all indicated. If the patient continues to have persistent signs and/or symptoms of ischemia, addition of a glycoprotein IIb/IIIa inhibitor should be considered.View Media Gallery

A 62-year-old woman with a history of chronic stable angina and a "valve problem" presents with new chest pain. She is symptomatic on arrival, complaining of shortness of breath and precordial chest tightness. Her initial vital signs are blood pressure = 140/90 mm Hg and heart rate = 98. Her electrocardiogram (ECG) is as shown. She is given nitroglycerin sublingually, and her pressure decreases to 80/palpation. Right ventricular ischemia should be considered in this patient.View Media Gallery
In difficult cases with nondiagnostic ECGs, such as those involving a left bundle-branch block, early imaging is useful to assess wall-motion abnormalities.
An important use of noninvasive imaging is to classify a patient has having NSTEMI or true STEMI.
The Optimal Cardiovascular Diagnostic Evaluation Enabling Faster Treatment of Myocardial Infarction (OCCULT-MI) trial compared the 80-lead (80L) mapping system to standard 12-lead (12L) ECG. The study concluded that the 80L body surface mapping technology detected more patients with MI or ACS than the 12L ECG, while still maintaining a high degree of specificity. [18]
A study by Damman et al examined information from 5,420 patients from the Fragmin and Fast Revascularization During Instability in Coronary Artery Disease (FRISC II), Invasive Versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS), and Randomized Intervention Trial of Unstable Angina 3 (RITA-3) patient-pooled database. The study found that admission ECG characteristics had long-term prognostic value for cardiovascular death or myocardial infarction. Quantitative ECG characteristics showed no incremental discrimination compared with qualitative data. [19]
A 5-year follow-up of patients with non–ST-elevation acute coronary syndrome from these 3 trials found no link between a procedure-related MI and long-term cardiovascular mortality. However, long-term mortality substantially increased after a spontaneous MI. [20]
Measurement of CK-MB Levels
CK-MB, the isoenzyme specific to the heart muscle, was the principal biomarker of cardiac injury until troponin supplemented it.
In the setting of myocardial infarction, plasma CK-MB concentrations typically rise about 4-6 hours after the onset of chest pain. They peak within 12-24 hours and return to baseline levels within 24-48 hours. Serial measurements obtained every 6-8 hours (at least 3 times) are warranted until peak values are determined.
The area under the concentration-time curve for CK-MB created with serial measurements of blood enzyme levels provides a reliable estimate of the size of the infarct.
Clinical settings other than ACS, such as trauma, heavy exertion, and skeletal muscle disease (eg, rhabdomyolysis), may elevate CK-MB values.
Determination of subforms of CK-MB (CK-MB2 that is more specific to heart muscle) may improve the sensitivity of this test.
Measurement of Troponin levels
The troponins are regulatory proteins found in skeletal and cardiac muscle. The three subunits that have been identified include troponin I (TnI), troponin T (TnT), and troponin C (TnC). The genes that code for the skeletal and cardiac isoforms of TnC are identical; thus, no structural difference exists between them. However, the skeletal and cardiac subforms for TnI and TnT are distinct, and immunoassays have been designed to differentiate between them. This explains the cardiospecificity of the cardiac troponins. Skeletal TnI and TnT are structurally different. No cross-reactivity occurs between skeletal and cardiac TnI and TnT with the current assays.
The cardiac troponins are sensitive, cardiospecific, and provide prognostic information for patients with ACS. They have become the cardiac markers of choice for patients with ACS.
Early studies on the release kinetics of the cardiac troponins indicated that they were not early markers of myocardial necrosis. The early generation troponin assays yielded positive results within 4-8 hours after symptom onset, similar in timing to the release of CK-MB; however, they remained elevated for as long as 7-10 days post-myocardial infarction.
Initial studies on the cardiac troponins revealed a subset of patients with rest unstable angina in whom CK-MB levels were normal but who had elevated troponin levels. These patients had higher adverse cardiac event rates (acute myocardial infarction, death) within the 30 days after the index admission and a natural history that closely resembled patients with NSTEMI. The table below outlines many of the initial studies on troponins in ACS.

Use of cardiac markers in the ED. Studies on troponins in ACS.View Media Gallery
As previously mentioned, an elevated troponin level also enables risk stratification of patients with ACS and identifies patients at high risk for adverse cardiac events (ie, myocardial infarction, death) up to 6 months after the index event. [5, 6]
In a study by Antman et al, the initial TnI level on admission in patients with ACS correlated with mortality at 6 weeks. CK-MB levels, although sensitive and specific for the diagnosis of acute myocardial infarction, were not predictive of adverse cardiac events and had no prognostic value. [5] The relationship between TnI levels and risk of cardiac events and mortality is demonstrated in the graphs below.
Use of cardiac markers in the ED. Troponin I levels and cardiac mortality in ACS.


Use of cardiac markers in the ED. Cardiac event rates in the platelet receptor inhibition for ischemic syndrome (PRISM) study based on troponin I results.
Data from a meta-analysis indicated that an elevated troponin level in patients without ST-segment elevation is associated with a nearly 4-fold increase in cardiac mortality rate. For the composite end point of acute myocardial infarction or death, an elevated troponin level was associated with an odds ratio of 3.3. [21]
The TIMI IIIB, GUSTO IIa, GUSTO IV ACS, and Fragmin During Instability in Coronary Artery Disease (FRISC) trial all demonstrated a direct correlation between the level of TnI or TnT and the adverse cardiac event rate and mortality rate in ACS. [5, 22, 23, 24, 25] These studies confirmed the use of the cardiac troponins TnI and TnT in risk stratification and therapeutic decision making.
Studies by Ohman et al and Stubbs et al revealed that an elevated troponin level at baseline was an independent predictor of mortality even in patients with chest pain and acute myocardial infarction with ST-segment elevation who were eligible for reperfusion therapy. [22, 26]
With the introduction of increasingly sensitive and precise troponin assays, up to 80% of patients with acute myocardial infarction will be found to have an elevated troponin within 2-3 hours of ED arrival. With this improved clinical performance in cardiac troponin assays, the so-called rapidly rising cardiac biomarkers, such as myoglobin or CK-MB isoforms, have little clinical utility. [27, 28, 29, 30] In a prospective multicenter study, patients with ACS who and presented with acute chest pain to the ED were followed for 12 months. The study found that patients with normal high-sensitivity cardiac troponin T (hs-cTnT) levels at presentation have low mortality rates but an increased rate of acute myocardial infarction during the subsequent 360 days. [31]
As a result, some authorities have called for a troponin standard alone and recommend eliminating CK-MB. [32]
Many patients with acute myocardial infarction present with equivocal ECG patterns, making prehospital ECG diagnosis difficult. A study by Sorensen et al suggests prehospital TnT testing may improve diagnosis in patients with chest pain transported by ambulance. [33] When quantitative TnT was measured at hospital arrival in 958 patients after 8 and 24 hours, a diagnosis of acute myocardial infarction was established in 208 of 258 patients with increased TnT levels, showing prehospital TnT testing is feasible with a high success rate. Prehospital implementation of quantitative tests, with lower detection limits, may identify most patients with acute myocardial infarction irrespective of ECG changes.
If myocardial injury is suspected despite negative cardiac-specific troponin findings, additional, sensitive laboratory assays are indicated. [34]
Patients with suspected ACS who test negative for troponin and copeptin can be safely discharged from the hospital without further testing, according to a recent study, the Biomarkers in Cardiology 8 (BiC-8) trial. Copeptin, a marker of severe hemodynamic stress, can be detected immediately in acute myocardial infarction. [35] The study involved 902 patients at low to intermediate risk of ACS; half of the patients were treated with standard care, and the other 451 patients underwent a copeptin assay. In the latter group, patients with a positive copeptin test, defined as a level of 10 pmol/L or greater, were treated with standard ACS care, while patients with a copeptin level below 10 pmol/L were discharged into ambulant care, including an outpatient visit within 72 hours. In the 451 patients tested for troponin and treated with standard care, the 30-day rate of major adverse cardiovascular events was 5.5%, compared with 5.46% in the 451 patients tested for troponin and copeptin (a statistically insignificant difference). [35]
Measurement of Myoglobin Levels
Myoglobin is not cardiac specific, but it may be detected as early as 2 hours after myocardial necrosis starts. However, myoglobin results should be supplemented with other, more specific cardiac biomarkers, such as CK-MB or troponin.
Myoglobin values have a high negative predictive value when blood is sampled in the first 4-8 hours after onset.
Complete Blood Count Determination
The CBC count helps in ruling out anemia as a secondary cause of ACS. Leukocytosis has prognostic value in the setting of acute myocardial infarction.
Basic Metabolic Panel
Obtain a basic metabolic profile, including a check of blood glucose level, renal function, and electrolytes levels, for patients with new-onset angina. Close monitoring of potassium and magnesium levels is important in patients with ACS because low levels may predispose them to ventricular arrhythmias. Routine measurement of serum potassium levels and prompt correction are recommended.
Creatinine levels must be considered before using an angiotensin-converting enzyme (ACE) inhibitor and particularly if cardiac catheterization is considered. Use of N -acetylcysteine and adequate hydration can help prevent contrast material–induced nephropathy. [36]
Other useful metabolic profiles include amylase and lipase.
A study by Charpentier et al suggests that a serum glucose level of more than 140 mg/dL is associated with non-ST elevation ACS in patients admitted to an ED for chest pain. However, when this level of blood glucose is added to the conventional diagnostic tools, the result is only a small increase in the ability to classify ACS. [37]
New Biomarkers
Levels of brain natriuretic peptide (BNP) and N-terminal pro-BNP (NT-pro-BNP) are elevated in acute MI and provide predictive information for risk stratification across the spectrum of ACS. [38, 39] However, a single, low BNP level obtained within 4 hours of a patient presenting to the ED does not identify the patient as low-risk for 30-day acute myocardial infarction or death. [40]
In the future, a combination of levels of troponin (a biomarker for myocardial necrosis), NT-pro-BNP (an indicator of elevated LV end-diastolic pressure and wall stress), and C-reactive protein (CRP, an estimate of the extent of systemic inflammation) may prove useful for predicting the outcome of patients with ACS.
Routine measurement of BNP and CRP levels in patients with ACS is not warranted at this time.
Interleukin-6 is the major determinant of acute-phase reactant proteins in the liver, and serum amyloid A is another acute-phase reactant. Elevations of either of these can be predictive in determining increased risk of adverse outcomes in patients with unstable angina.
Cavusoqlu et al suggests that elevated baseline levels of plasma interleukin-10 are associated with long-term adverse outcomes in patients with ACS. [41]
Several other biomarkers with variable sensitivity and specificity have been investigated, including sCD40 ligand, myeloperoxidase, pregnancy-associated plasma protein-A, choline, placental growth factor, cystatin C, fatty acid binding protein, ischemia modified albumin, chemokines ligand-5 and -18 (mediators of monocyte recruitment induced by ischemia), angiogenin, SCUBE1 (a novel platelet protein), and others. [42, 43] In a study that included 107 patients presenting to an emergency department with chest pain, ischemia modified albumin was not found to have superior sensitivity and specificity over traditional biomarkers, with a sensitivity of 0.86 and specificity of 0.49. [44]
Chest Radiography
Chest radiography helps in assessing cardiomegaly and pulmonary edema, or it may reveal complications of ischemia, such as pulmonary edema. It may also provide clues to alternative causes of symptoms, such as thoracic aneurysm or pneumonia (which can be a precipitating cause of ACS).
Echocardiography
Echocardiograms may play an important role in the setting of ACS. Regional wall-motion abnormalities can be identified with this modality, and echocardiograms are especially helpful if the diagnosis is questionable.
An echocardiogram can also help in defining the extent of an infarction and in assessing overall function of the left and right ventricles. In addition, an echocardiogram can help to identify complications, such as acute mitral regurgitation, LV rupture, and pericardial effusion.
Absence of segmental wall-motion abnormality on echocardiography during active chest discomfort is a highly reliable indicator of a nonischemic origin of symptoms, although echocardiography is of limited value in patients whose symptoms have resolved or who have pre-existing wall-motion abnormalities.
Myocardial Perfusion Imaging
Radionuclide myocardial perfusion imaging has been shown to have favorable diagnostic and prognostic value in the emergent setting, with an excellent early sensitivity in the detection of acute myocardial infarction not found in other testing modalities.
A normal resting perfusion imaging study has been shown to have a negative predictive value of more than 99% in excluding myocardial infarction. Observational and randomized trials of rest and stress imaging in the ED evaluation of patients with chest pain have demonstrated reductions in unnecessary hospitalizations and cost savings compared with routine care.
Perfusion imaging has also been used in risk stratification after myocardial infarction and for measurement of infarct size to evaluate reperfusion therapies. Novel "hot spot" imaging radiopharmaceuticals that visualize infarction or ischemia are currently undergoing evaluation and hold promise for future imaging of ACS.
Cardiac Angiography
Cardiac catheterization helps in defining coronary anatomy and the extent of a patient’s disease.
Patients with cardiogenic shock, intractable angina (despite medication), severe pulmonary congestion, or right ventricular (RV) infarction should immediately undergo cardiac catheterization. (Cardiogenic shock is defined as a systolic BP of less than 90 mm Hg in the presence of organ hypoperfusion.)
For high-risk patients with ACS without persistent ST elevation, angiography with glycoprotein IIb/IIIa inhibition has been recommended. The earlier that coronary angiography is performed, the lower the risk of recurrent ischaemia. [45] This also shortens the hospital stay for those patients.
Most patients benefit from angiography when they have a TIMI (Thrombolysis in Myocardial Infarction) risk score of less than 3 points (see Table 2, below).
Table 2. TIMI Risk Score for Unstable Angina and NSTEMI [46] (Open Table in a new window)
Characteristic
Risk Score
History
Age ≥65 years
1
At least 3 risk factors for coronary heart disease
1
Previous coronary stenosis ≥50%
1
Use of aspirin in previous 7 days
1
Presentation
At least 2 anginal episodes in the previous 24 hours
1
ST-segment elevation on admission ECG
1
Elevated levels of serum biomarkers
1
Total Score
0-7
Note: Event rates significantly increased as the TIMI risk score increased in the test cohort in the TIMI IIB study. Rates were 4.7% for a score of 0/1, 8.3% for 2, 13.2% for 3, 19.9% for 4, 26.2% for 5, and 40.9% for 6/7 (P< .001, χ2 test for the trend). The pattern of increasing event rates with increasing TIMI risk score was confirmed in all 3 validation groups (P< .001).
Computed Tomography Coronary Angiography and CT Coronary Artery Calcium Scoring
Dual-source 64-slice CT scanners can do a full scan in 10 seconds and produce high-resolution images that allow fine details of the patient's coronary arteries to be seen. This technology allows for noninvasive and early diagnosis of CAD and thus earlier treatment before the coronary arteries become more or completely occluded. It permits direct visualization of not only the lumen of the coronary arteries but also plaque within the artery. Dual-source 64-slice CT scanning is being used with intravenous (IV) contrast to determine if a stent or graft is open or closed.
CT coronary artery scoring is emerging as an attractive risk stratification tool in patients who are low risk for ACS. This imaging modality exposes the patient to very little radiation (1-2 msV). No contrast is needed, and the study does not have a requirement for heart rate. [47]
The CAPTURE study, a randomized diagnostic trial, compared the efficacy a comprehensive cardiothoracic CT examination in the evaluation of patients presenting to the emergency department with undifferentiated acute chest discomfort or dyspnea. [48] Comprehensive cardiothoracic CT scanning was reasonable, with a similar diagnostic yield to dedicated protocols, but it did not reduce the length of stay, rate of subsequent testing, or costs. The “triple rule out” protocol might be helpful in the evaluation of select patients, but these findings suggest that it should not be routinely used with the expectation that it will improve efficiency or reduce resource use.
Another study of the usefulness of CT for reducing hospital admission rates found that coronary computed tomographic angiography (CCTA) appears to allow safe, expedited discharge from the ED of low-to-intermediate-risk patients who would otherwise be admitted. [49]
Other Techniques
Optical coherence tomography (OCT), palpography, and virtual histology are being studied for use in identifying vulnerable plaques.
Noninvasive whole-blood test prior to coronary angioplasty may be useful for assessing obstructive CAD in patients without diabetes. [50]
Stress cardiac magnetic resonance imaging (MRI) in an observation unit setting has shown to reduce the medical costs, compared with inpatient care, for patients who present with emergent, non-low-risk chest pain, without missing acute coronary syndrome. [51]
The CAPTURE study, a randomized diagnostic trial, compared the efficacy a comprehensive cardiothoracic CT examination in the evaluation of patients presenting to the emergency department with undifferentiated acute chest discomfort or dyspnea. [52] Comprehensive cardiothoracic CT scanning was reasonable, with a similar diagnostic yield to dedicated protocols, but it did not reduce the length of stay, rate of subsequent testing, or costs. The “triple rule out” protocol might be helpful in the evaluation of select patients, but these findings suggest that it should not be routinely used with the expectation that it will improve efficiency or reduce resource use.
Treatment & Management
Approach Considerations
Initial therapy for ACS should focus on stabilizing the patient's condition, relieving ischemic pain, and providing antithrombotic therapy to reduce myocardial damage and prevent further ischemia. Morphine (or fentanyl) for pain control, oxygen, sublingual or intravenous (IV) nitroglycerin, soluble aspirin 162-325 mg, and clopidogrel with a 300- to 600-mg loading dose are given as initial treatment.
In complete vessel occlusion without collateralization of the infarct-related vessel, there is little utility in “pushing nitrates.”
High-risk patients with non-ST-segment elevation myocardial infarction (NSTEMI ACS) should receive aggressive care, including aspirin, clopidogrel, unfractionated heparin or low–molecular-weight heparin (LMWH), IV platelet glycoprotein IIb/IIIa complex blockers (eg, tirofiban, eptifibatide), and a beta blocker. The goal is early revascularization.
Intermediate-risk patients with NSTEMI ACS should rapidly undergo diagnostic evaluation and further assessment to determine their appropriate risk category.
Low-risk patients with NSTEMI ACS should undergo further follow-up with biomarkers and clinical assessment. Optimal medical therapies include use of standard medical therapies, including beta blockers, aspirin, and unfractionated heparin or LMWH. The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study showed that clopidogrel would be beneficial even in low-risk patients. [53] If no further pain occurs, and follow-up studies are negative, a stress study should drive further management.
Monitor and immediately treat arrhythmias in the first 48 hours. Pay attention to exacerbating factors, such as disturbances in electrolytes (especially potassium and magnesium), hypoxemia, drugs, or acidosis. Correct these factors accordingly.
Humidified oxygen may reduce the risk of nosebleeds in patients with ACS who are receiving antiplatelet and antithrombin therapy.
Do not administer nitrates if the patient is hypotensive (systolic BP < 90 mm Hg); if RV infarction, large pericardial effusion, or severe aortic stenosis is suspected; or if the patient recently received phosphodiesterase-5 inhibitors (eg, sildenafil).
Patients with known hypersensitivity to antiplatelet agents, active internal bleeding, and bleeding disorders should not receive antiplatelet or antithrombotic therapy.
Some patients with intractable chest pain or severe hypotension may require the insertion of an intra-aortic balloon pump. The EuroHeart survey showed a nearly 40% reduction in the risk of death in patients with ACS who received support with an intra-aortic balloon pump. This benefit was independent of the status of the ST segment.
Congestive heart failure (CHF) can be due to systolic dysfunction or diastolic dysfunction in the setting of myocardial infarction. Aggressive treatment is indicated to prevent worsening of the situation.
Patients presenting with cardiogenic shock should undergo percutaneous coronary intervention (PCI) as soon as possible. Cardiogenic shock is associated with a high mortality rate. Pressor agents, such as dopamine, and inotropic agents, such as dobutamine, may be needed. In a prospective, natural-history study of coronary atherosclerosis, patients underwent 3-vessel coronary angiography and gray-scale and radiofrequency intravascular ultrasonographic imaging after PCI. [54]
Recurrent ischemia may be due to incomplete reperfusion. In the setting of PCI, consider stent thrombosis as a possible cause. Whether drug-eluting stents have an increased rate of thrombosis compared with bare metal stents is unclear.
The clinical significance of incomplete coronary revascularization (ICR) following PCI in patients with ACS was examined in 2,954 patients from the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. At one year follow-up, ICR was strongly associated with ischemia-driven unplanned revascularization, myocardial infarction and major adverse cardiac events. [55]
Drug-eluting stents are linked with fewer periprocedural risks but tend to have high incidence of postprocedural complications including myocardial infarction, repeat procedures, and 12-month major adverse cardiac and brain complications, compared with coronary bypass surgery. [56]
One study by Ribichini et al suggests that prednisone treatment after bare metal stents or drug-eluting stent implantation results in a better event-free survival at 1 year. [57]
In the final report of the HORIZONS-AMI trial, which assessed the 3 year outcomes of the effectiveness and safety of bivalirudin monotherapy and paclitaxel-eluting stenting, outcomes were sustained for patients with STEMI undergoing primary PCI. [58]
In a study of 3031 patients, Mehta et al found that early intervention (coronary angiography ≤24 h after randomization) in patients with ACS did not differ greatly from delayed intervention (coronary angiography ≥36 h randomization) in the prevention of primary outcomes (ie, composite of death, myocardial infarction, or stroke at 6 mo). Early intervention did reduce the rate of secondary outcomes (ie, death, myocardial infarction, or refractory ischemia at 6 mo) and improved primary outcomes in patients who were at highest risk (ie, GRACE risk score >140). [59]
In a Swedish registry of patients with STEMI from 1996-2007, reported an increase in the prevalence of evidence-based treatments. [60] The use of aspirin, clopidogrel, beta blockers, statins, and ACE inhibitors all increased. Clopidogrel increased from 0% to 82%, statins increased from 23% to 83%, and various ACE inhibitors increased by a large margin. A decrease was reported in 30-day and 1-year mortality that was sustained during long-term follow-up. By following the proper guidelines, patients who have experienced STEMI have higher survival rates.
Pharmacologic Anti-ischemic Therapy
Nitrates
Nitrates do not improve mortality. [61] However, they provide symptomatic relief by means of several mechanisms, including coronary vasodilation, improved collateral blood flow, decrease in preload (venodilation and reduced venous return), and decrease in afterload (arterial vasodilation). Care should be taken to avoid hypotension, because this can potentially reduce coronary perfusion pressure (diastolic BP - LV diastolic pressure).
Beta-blockers
Beta-blockers are indicated in all patients unless they have the following contraindications:
Systolic blood pressure less than 90 mm Hg
Cardiogenic shock
Severe bradycardia
Second- or third-degree heart block
Asthma or emphysema that is sensitive to beta agonists
Peripheral vascular disease
Uncompensated CHF
Beta blockers reduce oxygen demand and ventricular wall tension. They also decrease mortality and adverse cardiovascular events. These drugs may prevent mechanical complications of myocardial infarction, including rupture of the papillary muscle, left ventricular free wall, and ventricular septum. Beta blockers meliorate dynamic obstruction of the left ventricular outflow tract in patients with apical infarct and hyperdynamic basal segments.
The most frequently used regimen is IV metoprolol 2-5 mg given every 5 minutes (up to 15 mg total) followed by 25-100 mg given orally twice a day.
Beta-blockers should not be used acutely in patients with cardiogenic shock or signs of heart failure on presentation.
Pharmacologic Antithrombotic Therapy
Aspirin
Aspirin permanently impairs the cyclooxygenase pathway of thromboxane A2 production in platelets, in this way inhibiting platelet function. Aspirin reduces morbidity and mortality and is continued indefinitely. [62]
Clopidogrel
Clopidogrel (thienopyridine) inhibits adenosine 5'-diphosphate (ADP)–dependent activation of the glycoprotein IIb/IIIa complex, a necessary step for platelet aggregation. This process results in intense inhibition of platelet function, particularly in combination with aspirin. In the CURE trial, thienopyridine reduced the rate of myocardial infarction by 20%. [53]
The optimal loading dose for clopidogrel is still being evaluated. Reports show that a loading dose of 600 mg might be more beneficial than 300 mg. Withhold clopidogrel for at least 5 days before elective coronary artery bypass grafting (CABG). Since 12% of patients with non-ST elevation ACS have coronary anatomy that favors CABG, the use of clopidogrel is withheld until coronary angiography at some institutions.
A meta-analysis of 34 studies analyzed the safety of CABG among patients with ACS continuing clopidogrel. The investigators found that although mortality is increased in those receiving clopidogrel, it is influenced by ACS status and case urgency in primarily nonrandomized studies. Among patients with ACS, no differences in mortality or postoperative myocardial infarction or stroke rates were found. This suggests that in patients with ACS who require urgent CABG, proceeding despite the continuation of clopidogrel is likely safe. [63]
Clopidogrel can be considered an alternative to aspirin in patients with aspirin intolerance or who are allergic to aspirin.
Patients with chronic kidney disease who have low platelet response to clopidogrel tend to have worse outcomes after PCI. [64]
Evidence has shown that dexlansoprazole and lansoprazole do not significantly reduce the conversion of clopidogrel to its active metabolite (reduced by 9% and 14%, respectively), and no dose adjustment of clopidogrel is required. [65, 66]
The group’s findings and recommendations are listed below.
Clopidogrel reduces major CV events compared with placebo or aspirin.
Dual antiplatelet therapy with clopidogrel and aspirin, compared with aspirin alone, reduces major CV events in patients with established ischemic heart disease, and it reduces coronary stent thrombosis but is not routinely recommended for patients with prior ischemic stroke because of the risk of bleeding. [67]
Clopidogrel alone, aspirin alone, and their combination are all associated with increased risk of GI bleeding.
Clopidogrel requires metabolic activation by cytochrome P450 2C19 (CYP2C19). PPIs that inhibit CYP2C19 are commonly coadministered with clopidogrel to reduce the risk of GI bleeding. A study by Simon et al showed that PPI use is not associated with an increased risk of cardiovascular events or mortality in patients who have been treated with clopidogrel for a recent MI, regardless of CYP2C19 genotype. [68]
Patients with prior GI bleeding are at highest risk for recurrent bleeding on antiplatelet therapy; other risk factors include advanced age, concurrent use of anticoagulants, steroids, or NSAIDs including aspirin, and Helicobacter pylori infection; risk increases as the number of risk factors increases.
Use of PPIs or histamine H2 receptor antagonists (H2RAs) reduces the risk of upper GI bleeding compared with no therapy; PPIs reduce upper GI bleeding to a greater degree than do H2Ras.
PPIs are recommended to reduce GI bleeding among patients with a history of upper GI bleeding; PPIs are appropriate in patients with multiple risk factors for GI bleeding who require antiplatelet therapy.
Routine use of either a PPI or an H2RA is not recommended for patients at lower risk of upper GI bleeding, who have much less potential to benefit from prophylactic therapy.
Clinical decisions regarding concomitant use of PPIs and thienopyridines must balance overall risks and benefits, considering both CV and GI complications.
Pharmacokinetic and pharmacodynamic studies, using platelet assays as surrogate endpoints, suggest that concomitant use of clopidogrel and a PPI reduces the antiplatelet effects of clopidogrel; the strongest evidence for an interaction is between omeprazole and clopidogrel; it is not established that changes in these surrogate endpoints translate into clinically meaningful differences.
Observational studies and a single randomized clinical trial have shown inconsistent effects on CV outcomes of concomitant use of thienopyridines and PPIs; a clinically important interaction cannot be excluded, particularly in certain subgroups, such as poor metabolizers of clopidogrel.
The role of either pharmacogenomic testing or platelet function testing in managing therapy with thienopyridines and PPIs has not yet been established.
Prasugrel
Like clopidogrel, prasugrel is a thienopyridine ADP receptor inhibitor that inhibits platelet aggregation. It has been approved in the United States and has been shown to reduce new and recurrent myocardial infarctions. [69] The loading dose is 60 mg PO once and maintenance is 10 mg PO qd (given with aspirin 75-325 mg/d). Prasugrel is indicated for the reduction of thrombotic cardiovascular events (including stent thrombosis) with ACS that is managed with PCI.
However, a 2014 report by Montalescot et al indicates that if angiography is to be performed in a patient with NSTEMI within 48 hours of admission, treatment with the P2Y12 antagonist prasugrel should be postponed until a decision about revascularization has been reached owing to an increased bleeding risk without ischemia benefit from pre-PCI prasugrel therapy. [70, 71] The randomized, double-blind study included 2770 NSTEMI patients who underwent percutaneous coronary intervention (PCI), with 1394 patients receiving pretreatment with prasugrel and 1376 individuals receiving placebo. All patients received prasugrel at the time of PCI.
The investigators found that the same percentage of patients (13.1%) in the pretreatment and placebo groups reached primary endpoint (defined as time to first occurrence of glycoprotein IIb/IIIa bailout, stroke, myocardial infarction [MI], urgent revascularization, or cardiovascular death, through 7 days after randomization). [70, 71] No reductions in ischemic events, including total mortality, were found in the patients who received prasugrel pretreatment. Moreover, pretreatment with prasugrel was associated with a six-fold increase in life-threatening bleeding unrelated to coronary artery bypass grafting (CABG), as well as a three-fold increase in all major bleeding associated with non-CABG thrombolysis in myocardial infarction (TIMI). An approximately three-fold increase in TIMI minor bleeding events was also seen. [70, 71]
Earlier studies also found that significant, sometimes fatal, bleeding occurred more frequently with prasugrel than with clopidogrel, although the overall mortality rate did not differ significantly between a treatment group receiving prasugrel and another receiving clopidogrel. [69, 72]
In a separate, earlier study of patients with unstable angina or myocardial infarction without ST-segment elevation, prasugrel, compared with clopidogrel, did not significantly reduce the frequency of the primary end point of death from cardiovascular causes, myocardial infarction, or stroke. Similar bleeding risks were observed. [73]
Vorapaxar
In May 2014, the FDA approved vorapaxar (Zontivity) to reduce the risk of MI, stroke, cardiovascular death, and need for revascularization procedures in patients with a previous MI or peripheral artery disease (PAD). It is a first-in-class antiplatelet medication that is a protease-activated receptor 1 (PAR-1) inhibitor. It is not indicated as monotherapy, but in addition to aspirin and/or clopidogrel.
Approval was based on the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial. Results of the trial (n = 26,499) showed that time to cardiovascular death, MI, stroke, or urgent coronary revascularization was decreased by 13% in patients taking vorapaxar. When coronary revascularization was excluded, the secondary endpoint of cardiovascular death, MI, or stroke was also significantly reduced. [74]
Because of vorapaxar’s antiplatelet effects, moderate or severe bleeding occurred in 3.4% of patients compared with 2.1% in the placebo-treated patients. Intracranial hemorrhage occurred in 0.6% of those taking vorapaxar compared with 0.4% taking placebo. [74]
Ticagrelor
Ticagrelor (Brilinta) was approved by the US Food and Drug Administration in July 2011 and is the first reversible oral P2Y receptor antagonist. Results from the randomized PLATO (PLATelet inhibition and patient Outcomes) trial showed that ticagrelor provides faster, greater, and more consistent ADP-receptor inhibition than clopidogrel. [75]
In the PLATO trial, the difference between treatments on the composite resulted from effects on CV death and MI; each was statistically significant when considered as a secondary endpoint, and there was no beneficial effect on strokes. [75, 76, 77] For all-cause mortality, the benefit was also statistically significant of 9.8% for ticagrelor and 11.7% for clopidogrel (P = 0.0003) with a hazard ratio of 0.78.
Bleeding risk was assessed in the PLATO trial, and ticagrelor increased the overall risk of bleeding (major + minor) to a somewhat greater extent than did clopidogrel. [75] The increase was seen for non-CABG-related bleeding but not for CABG-related bleeding. Fatal and life-threatening bleeding rates were not increased.
In September 2015, the indication for ticagrelor was expanded to include use in patients with a history of MI more than 1 year previously. [78] Approval is based on the PEGASUS TIMI-54 study, a large-scale outcomes trial involving over 21,000 patients. [79] PEGASUS TIMI-54 investigated ticagrelor 60 mg twice daily plus low-dose aspirin, compared to placebo plus low-dose aspirin, for the long-term prevention of CV death, heart attack, and stroke in patients who had experienced an MI 1-3 years prior to study enrollment. In patients with an MI more than 1 year previously, treatment with ticagrelor significantly reduced the risk of CV death, MI, or stroke compared with placebo. [79]
Prevention of stent thrombosis
A subgroup analysis of the PLATO trial indicated that treatment with ticagrelor resulted in a lower risk of stent thrombosis than treatment with clopidogrel in patients with ACS. [80, 81] This benefit was independent of patient characteristics at baseline, including type of ACS and stent type.
Of the 18,624 ACS patients in the PLATO study, 11,289 (61%) had a previous stent implanted or received one during the trial. [81] Of these, 177 patients (1.6%) had a definite stent thrombosis (176 of them within 1 year), and 275 (2.5%) had definite or probable stent thrombosis.
Definite stent thrombosis occurred in 1.37% of patients treated with ticagrelor and 1.93% of those treated with clopidogrel—a 33% reduction in risk with ticagrelor. [81] Definite or probable stent thrombosis occurred in 2.21% of ticagrelor-treated patients and 2.87% of those who received clopidogrel—a 25% reduction in risk with ticagrelor. Overall, the risk of definite, probable, or possible stent thrombosis was reduced by 23% with ticagrelor.
Abciximab, eptifibatide, and tirofiban
Glycoprotein IIb/IIIa receptor antagonists include abciximab, [82, 83] eptifibatide, [84] and tirofiban. [85] These drugs inhibit the glycoprotein IIb/IIIa receptor, which is involved in the final common pathway for platelet adhesion and aggregation. (See the image below.)
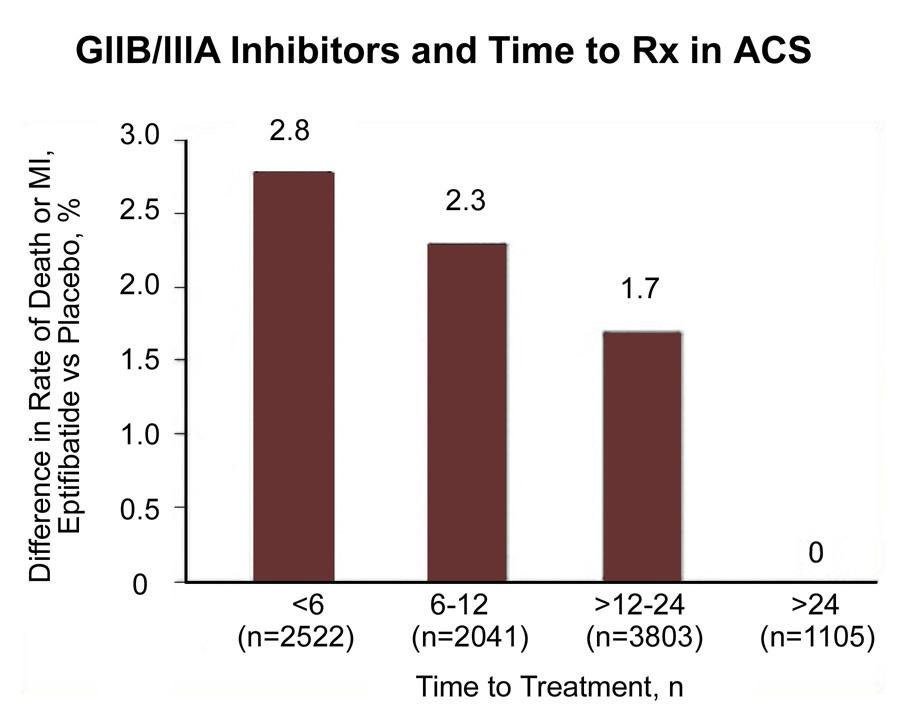
Use of cardiac markers in the ED. Effect of time to treatment in patients with acute coronary syndrome (ACS) who are treated with the GIIb/IIIa inhibitor eptifibatide.View Media Gallery
Use eptifibatide or tirofiban in patients with high-risk features in whom invasive treatment is not planned.
The use of eptifibatide 12 hours or more before angiography was not superior to the provisional use of eptifibatide after angiography, according to results from the EARLY ACS trial. The study compared a strategy of early, routine administration of eptifibatide with delayed, provisional administration in patients who had ACS without ST-segment elevation and who were assigned to an invasive strategy. The study also found that early use of eptifibatide was associated with an increased risk of non–life-threatening bleeding and the need for transfusion. [86]
Two trials with tirofiban and 1 trial with eptifibatide documented their efficacy in unstable angina/NSTEMI patients, only some of whom underwent interventions. These antagonists are a class I recommendation in patients in whom catheterization and PCI are planned. Intermediate- and high-risk patients appear to respond favorably to glycoprotein IIb/IIIa inhibitors. [87] They include patients with ST-segment depression, elevated risk scores, elevated serum troponin levels, [88] and/or diabetes mellitus.
Currently, IIb/IIIb antagonists in combination with aspirin are considered standard antiplatelet therapy for patients at high risk for unstable angina.
Pharmacologic Anticoagulation Therapy
Unfractionated heparin
A study by Oler et al found that unfractionated heparin was associated with a 33% reduction in the risk of myocardial infarction or death in patients with unstable angina who were treated with aspirin plus heparin, compared with patients who were treated with aspirin alone. [89] The FUTURA/OASIS-8 randomized trial found that low-dose unfractionated heparin, 50 U/kg (regardless of use of glycoprotein IIb/IIIa inhibitors), compared with standard-dose unfractionated heparin, 85 U/kg (60 U/kg with Gp IIb/IIIa inhibitors), did not reduce major peri-PCI bleeding and vascular access-site complications. [90]
Low–molecular-weight heparin
LMWHs might be superior to unfractionated heparin in reducing cardiovascular outcomes, with a safety profile similar to that of heparin in patients receiving medical care.
Conflicting results emerged from 9 randomized trials directly comparing LMWH with unfractionated heparin. Two trials evaluated dalteparin, another evaluated nadroparin, and 6 evaluated enoxaparin. [91, 92] Trials with dalteparin and nadroparin reported similar rates of nonfatal myocardial infarction or death compared with heparin, whereas 5 of 6 trials of enoxaparin found point estimates for death or nonfatal myocardial infarction that favored enoxaparin over heparin. The benefit of enoxaparin appeared to be driven largely by a reduction in nonfatal myocardial infarction, especially in the cohort of patients who had not received any open-label anticoagulant therapy before randomization.
In addition, a systematic review comparing LMWH with unfractionated heparin found no significant difference in benefits between the two drugs.
Aside from the possible medical benefits of using LMWH in place of unfractionated heparin, advantages of LMWH include ease of administration, absence of need for anticoagulation monitoring, and potential for overall cost savings. Although three LMWHs are approved for use in the United States, only enoxaparin is currently approved for use in unstable angina. Lev et al found that the combination of eptifibatide with enoxaparin appears to have a more potent antithrombotic effect than that of eptifibatide and unfractionated heparin. [93]
The role of LMWHs in patients for whom PCI is scheduled is relatively ill defined. However, it is likely to be at least equivalent to that of heparin. It appears reasonable to minimize the risk of excessive anticoagulation during PCI by avoiding crossover of anticoagulants (ie, maintain consistent anticoagulant therapy from the pre-PCI phase throughout the procedure itself). Additional experience with regard to the safety and efficacy of the concomitant administration of LMWHs with Gp IIb/IIIa antagonists and fibrinolytic agents is currently being acquired.
Adding apixaban (5 mg twice daily) to antiplatelet therapy in high-risk patients after ACS may increase the number of major bleeding events without significantly reducing recurrent ischemic events. [94]
Factor Xa inhibitors
Use of the oral Xa inhibitor rivaroxaban in patients with ACS was investigated in the ATLAS ACS 2-TIMI 51 trial. [95, 96] Cutting the rivaroxaban dosage from 5 mg twice daily to 2.5 mg twice daily reduced deaths and bleeding after ACS. Mortality from recurrent events was significantly lower with the 2.5-mg dose (30.6% vs 43.8%), as was the death rate from new MI (8.8% vs 17.2%). The risk of fatal bleeding was 61% lower with the 2.5-mg dose, and rates of TIMI bleeding requiring medical attention were significantly lower (12.9% vs 16.2%).
Another factor Xda inhibitor, fondaparinux (Arixtra), has been studied for use in patients with STEMI who do not undergo PCI. [97] In the Fifth Organization to Assess Strategies in Ischemic Syndromes (OASIS-5) trial, fondaparinux reduced major bleeding and improved net clinical outcome compared with enoxaparin in patients receiving GP IIb/IIIa inhibitors or thienopyridines for ACS. [98] Fondaparinux is not currently FDA approved for use in ACS.
Thrombolysis
Prehospital thrombolysis allows eligible patients to receive thrombolysis 30-60 minutes sooner than if treatment were given in the ED; however, prehospital thrombolysis is still under investigation and has not become a trend, as a result of unproven benefit and an increase in the availability of PCI in many medical centers as an alternative to thrombolysis for STEMI.
The Remodeling With Erythropoietin After Large Myocardial Infarction (REVEAL) trial evaluated the safety and efficacy of a single intravenous bolus of epoetin alfa in patients with STEMI who had successful reperfusion with primary or rescue PCI. [99] A single intravenous bolus of epoetin alfa within 4 hours of PCI did not reduce infarct size and was associated with higher rates of adverse cardiovascular events.
Although PCI is the preferred treatment for STEMI, the distance to primary PCI centers and the inherent time delay in delivering primary PCI limits widespread use of this treatment. Prehospital electrocardiographic (ECG) diagnosis and direct referral for primary PCI enables patients with STEMI living far from a PCI center to achieve a system delay comparable to patients who are closer to a PCI center. [100]
Coronary Interventions
An early invasive strategy (ie, diagnostic angiography with intent to perform revascularization) is indicated in unstable angina/NSTEMI patients who have refractory angina or hemodynamic or electrical instability without serious comorbidities or contraindications to such procedures. [32] An early invasive strategy is also indicated in initially stabilized unstable angina/NSTEMI patients who do not have serious comorbidities or contraindications to such procedures and who have an elevated risk for clinical events.
In NSTEMI ACS, early revascularization reduces myocardial infarction and death rates compared with a more selective strategy, particularly in high-risk patients. Use of Gp IIb/IIIa blockers followed by early invasive catheterization is the most logical approach. An early invasive strategy should be considered in patients with large myocardial infarction, hypotension, shock, RV infarction, and refractory chest pain.
In the Invasive Versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) trial, an early invasive strategy had no apparent long-term benefit in reducing death or myocardial infarction. After stratification for risk, analysis of 5-year clinical outcomes in patients presenting with non-ST-segment elevation ACS and elevated troponin T (TnT) level showed that cumulative myocardial infarction or death rates were 22.3% in the early invasive group versus 18.1% in the selective invasive group. No difference was observed in mortality or myocardial infarction. [101]
Concomitant Therapy
Current guidelines for patients with moderate- or high-risk ACS recommend an early invasive approach with concomitant antithrombotic therapy, including aspirin, clopidogrel, and unfractionated or LMWH. The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial evaluated the role of thrombin-specific anticoagulation with bivalirudin in this patient population. In patients with moderate- or high-risk ACS who were undergoing invasive treatment with glycoprotein IIb/IIIa inhibitors, bivalirudin was associated with rates of ischemia and bleeding that were similar to those with heparin. Bivalirudin alone was associated with similar rates of ischemia and significantly lower rates of bleeding. [102] Further, glycoprotein IIb/IIIa inhibitors can be initiated at the time of angiography; routine administration 12-24 hours before the procedure carries an increased risk of bleeding and no improvement in outcome.
Kastrati et al compared the combination of glycoprotein IIb/IIIa inhibitors and heparin with bivalirudin, specifically among patients with NSTEMI undergoing PCI. In a double-blind manner, 1721 patients with acute NSTEMI were randomly assigned to receive abciximab plus unfractionated heparin (861 patients) or bivalirudin (860 patients). The study concluded that abciximab and unfractionated heparin, compared with bivalirudin, failed to reduce death, large recurrent myocardial infarction, urgent target-vessel revascularization, or major bleeding within 30 days. It also increased the risk of bleeding among patients with NSTEMI who were undergoing PCI. [103]
Guidelines Summary
Chronic coronary syndromes clinical practice guidelines (2019)
In August 2019, the European Society of Cardiology (ESC) released updated recommendations to their 2013 guidelines for the diagnosis and management of chronic coronary syndromes (CCS) (formerly stable coronary artery disease [CAD]). [104, 105]
The ESC introduced the term CCS in 2018 for what has been known as stable CAD to bring the terminology more in line with contemporary understanding of its development, progression, and management. The ESC indicates that “the clinical presentations of CAD can be categorized as either acute coronary syndrome (ACS) or CCS,” and that “CAD is a dynamic process” of atherosclerosis and altered arterial function “that can be modified by lifestyle, pharmacological therapies, and revascularization, which result in disease stabilization or regression.” CCS is also viewed as a type of out-of-hospital counterpart to ACS.
The updated guidelines define six clinical scenarios that reflect the heterogeneous nature of CCS, each defined by its own set of diagnostic and therapeutic concerns, as follows:
Suspected CAD with "stable" anginal symptoms, with or without dyspneaSuspected CAD with new-onset heart failure symptoms or left ventricular (LV) dysfunctionAsymptomatic or stabilized symptomatic within 1 year of an ACS episode or following recent coronary revascularizationAsymptomatic or symptomatic more than 1 year after the initial diagnosis or revascularizationAngina and suspected vasospastic or microvascular diseaseAsymptomatic with CAD detected at screening
The new phrase “clinical likelihood of CAD” uses various CAD risk factors as modifiers of pretest probability (PTP).
New Recommendations
Basic testing, diagnostics, and risk assessment
It is recommended that the initial test for diagnosing CAD in symptomatic patients in whom obstructive CAD cannot be ruled out based on clinical assessment alone be noninvasive functional imaging for myocardial ischemia or coronary computed tomography angiography (CTA). If coronary CTA reveals CAD of uncertain functional significance or is not diagnostic, functional imaging for myocardial ischemia is recommended.
The choice of the initial noninvasive diagnostic test is based on the clinical likelihood of CAD as well as other patient characteristics that influence test performance, local expertise, and the availability of tests.
Invasive angiography is recommended as an alternative test to diagnose CAD in patients with a high clinical likelihood and severe symptoms refractory to medical therapy, or typical angina at a low level of exercise and clinical evaluation that indicates high event risk. Invasive functional assessment must be available and used to evaluate stenoses before revascularization, unless the stenoses are very high grade (>90% diameter stenosis).
Consider the use of invasive coronary angiography with the availability of invasive functional evaluation to confirm the diagnosis of CAD in patients with an uncertain diagnosis on noninvasive testing.
Consider coronary CTA as an alternative to invasive angiography if another noninvasive test is equivocal or nondiagnostic. However, coronary CTA is not recommended in the setting of extensive coronary calcification, irregular heart rate, significant obesity, inability to cooperate with breath-hold commands, or any other conditions that would make good image quality unlikely.
When screening for CAD in asymptomatic patients, carotid ultrasound intima-media thickness (IMT) is not recommended for cardiovascular (CV) risk assessment.
Antithrombotic therapy
In patients with CCS and sinus rhythm, consider the addition of a second antithrombotic drug to aspirin for long-term secondary prevention in patients with a high risk of ischemic events and without a high bleeding risk. This drug regimen may be considered in those with at least a moderately increased risk of ischemic events and without a high bleeding risk.
In patients with CCS and atrial fibrillation (AF) in whom oral anticoagulation (OAC) is initiated and who are eligible for a non-vitamin K antagonist OAC (NOAC), NOAC is preferred to a vitamin K antagonist (VKA). Long-term OAC therapy (a NOAC or VKA with time in the therapeutic range >70%):
Is recommended in patients with AF and a CHA 2DS 2- VASc score of at least 2 in males and at least 3 in females (CHA 2DS 2- VASc: C ardiac failure, H ypertension, A ge ≥75 [doubled], D iabetes, S troke [doubled], V ascular disease, A ge 65-74, Sex [female])Should be considered in patients with AF and a CHA 2DS 2- VASc score of 1 in males and 2 in females
In post-percutaneous coronary intervention (PCI) patients with AF or another indication for OAC:
In those eligible for a NOAC, NOAC (apixaban 5 mg bid, dabigatran 150 mg bid, edoxaban 60 mg od, or rivaroxaban 20 mg od) is preferred to a VKA in combination with antiplatelet therapy.When rivaroxaban is used and concerns about high bleeding risk outweigh those about stent thrombosis or ischemic stroke, consider rivaroxaban 15 mg od over rivaroxaban 20 mg od for the duration of the concomitant single or dual antiplatelet therapy (DAPT).When dabigatran is used and concerns about high bleeding risk outweigh those about stent thrombosis or ischemic stroke, consider dabigatran 110 mg bid over dabigatran 150 mg bid for the duration of the concomitant single or dual antiplatelet therapy.After uncomplicated PCI, consider early aspirin cessation (≤1 week), and continuation of dual therapy with OAC and clopidogrel, if there is a low risk of stent thrombosis or if concerns about bleeding risk outweigh those about the risk of stent thrombosis, irrespective of the type of stent used.Consider triple therapy with aspirin, clopidogrel, and an OAC for at least 1 month when the risk of stent thrombosis outweighs the bleeding risk, with the total duration (≤6 months) decided upon according to the assessment of these risks and clearly specified at hospital discharge.In patients with an indication for a VKA in combination with aspirin and/or clopidogrel, carefully regulate the VKA dose intensity with a target international normalized ratio (INR) in the range of 2.0-2.5 and with time in the therapeutic range above 70%.In patients with a moderate or high risk of stent thrombosis, irrespective of the type of stent used, dual therapy with an OAC and either ticagrelor or prasugrel may be considered as an alternative to triple therapy with an OAC, aspirin, and clopidogrel.
Other pharmacotherapy
Concomitant use of a proton pump inhibitor is recommended in patients receiving aspirin monotherapy, DAPT, or OAC monotherapy who are at high risk of gastrointestinal bleeding.
Lipid-lowering drugs:
If goals are not achieved with the maximum tolerated statin dose: Combination with ezetimibe is recommended.For patients at very high risk who do not achieve their goals on a maximum tolerated dose of statin and ezetimibe: Combination with a PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor is recommended.
Consider angiotensin-converting enzyme inhibitors in CCS patients at very high risk of CV adverse events.
In patients with diabetes mellitus and CV disease (CVD):
The sodium-glucose co-transporter 2 inhibitors empagliflozin, canagliflozin, or dapagliflozin are recommended.A glucagon-like peptide-1 receptor agonist (liraglutide or semaglutide) is recommended.
Treatment options for refractory angina
A reducer device for coronary sinus constriction may be considered to ameliorate symptoms of debilitating angina refractory to optimal medical and revascularization strategies.
For more information, please go to Primary and Secondary Prevention of Coronary Artery Disease, Acute Coronary Syndrome, and Atrial Fibrillation.
Screening
In 2015, the American College of Physicians (ACP) released guidelines on screening for coronary heart disease, including the following [15] :
There is no evidence that cardiac screening improves patient outcomes in asymptomatic, low-risk adults.Potential harms of cardiac screening include false-positive results causing patients to undergo potentially unnecessary tests and procedures.Among adults at low risk, prevalence of coronary heart disease is low, and cardiac screening is of low predictive value. Therefore, cardiac screening is of low yield, and the probability that positive findings will influence therapeutic decision making is low.Clinicians should therefore emphasize strategies to reduce cardiovascular risk even further among low-risk adults by treating modifiable risk factors (smoking, diabetes, blood pressure, hyperlipidemia, overweight, and exercise).Clinicians should not screen asymptomatic, low-risk adults for cardiac disease using resting or stress electrocardiography, stress echocardiography, or stress myocardial perfusion imaging.Clinicians should conduct cardiovascular risk assessment with a global risk score combining individual risk factor measurements into a single quantitative estimate of risk.The ACP recommendations do not apply to symptomatic patients or to screening athletes before participation in various events.
Management guidelines
The American College of Cardiology in conjunction with with the American Heart Association (AHA/ACC) and the European Society of Cardiology (ESC) have developed guidelines for management of patients with ST-segment elevation myocardial infarction (STEMI) [106, 107, 108, 109] and non-ST-elevation acute coronary syndromes (NSTE-ACS) [1, 110] which focus primarily on treatment in a hospital setting. Guidelines with specific recommendations for prehospital and emergency department (ED) management of patients with ACS were included in the 2015 revised international consensus guidelines on cardiopulmonary resuscitation and emergency cardiovascular care issued by the International Liaison Committee on Resuscitation (ILCOR). [111]
Evaluation
The 2015 ILCOR recommendations for diagnostic interventions in ACS include the following:
Prehospital 12-lead electrocardiographic (ECG) acquisition with hospital notification should be obtained for adult patients with suspected STEMI (strong recommendation, low-quality evidence).Computer-assisted ECG interpretation can be used as an adjunct to identify STEMI (weak recommendation, very-low-quality evidence), but it should not be used alone to rule out STEMI because of the poor sensitivity of the computer algorithms evaluated (weak recommendation, very-low-quality evidence).For patients with suspected STEMI outside of a hospital setting, nonphysicians may perform ECG interpretation to recognize STEMI in a system where the false-positive (FP) and false-negative (FN) rates are low (weak recommendation, very-low-quality evidence).When primary percutaneous coronary intervention (PPCI) is the planned strategy, prehospital activation of the catheterization laboratory for PPCI is preferred (strong recommendation, very-low-quality evidence).The use of troponins at 0 and 2 hours as a stand-alone measure for excluding the diagnosis of ACS is strongly discouraged (strong recommendation, very-low-quality evidence). Excluding the diagnosis of ACS can be accomplished by combining negative high-sensitivity cardiac troponin (hs-cTnI) measured at 0 and 2 hours with low-risk stratification or by combining negative cardiac troponin I (cTnI) or cardiac troponin T (cTnT) measured at 0 and 3 to 6 hours with very low risk stratification (weak recommendation, low-quality evidence).
The 2014 AHA/ACC revision of their 2007 guidelines for the management of NSTE-ACS includes the following recommendations for evaluation of patients with suspected ACS, as summarized below. [110]
Class I
Risk stratify patients with suspected ACS based on the likelihood of ACS and adverse outcome(s) to decide on the need for hospitalization and to assist in the selection of treatment options. (Level of evidence: B)Immediately refer patients with suspected ACS and high-risk features (eg, continuing chest pain, severe dyspnea, syncope/presyncope, or palpitations) to the emergency department (ED) and transport by emergency medical services when available. (Level of evidence: C)In patients with chest pain or other symptoms suggestive of ACS, perform a 12-lead ECG and evaluate for ischemic changes within 10 minutes of the patient’s arrival at an emergency facility when possible. (Level of evidence: C)Perform serial ECGs (eg, 15- to 30-minute intervals during the first hour) to detect ischemic changes if the initial ECG is not diagnostic but the patient remains symptomatic. (Level of evidence: C)Obtain serial cardiac troponin I or T levels (when a contemporary assay is used) at presentation and 3 to 6 hours after symptom onset in all patients who present with symptoms consistent with ACS (to identify a rising and/or falling pattern of values). If the time of symptom onset is ambiguous, the time of presentation should be considered the time of onset for assessing troponin values. (Level of evidence: A)Obtain additional troponin levels beyond 6 hours after symptom onset in patients with normal troponin levels on serial examination when changes on ECG and/or clinical presentation confer an intermediate or high index of suspicion for ACS. (Level of evidence: A)
Class IIa
It is reasonable to give low-risk patients who are referred for outpatient testing daily aspirin, short-acting nitroglycerin, and other medications if appropriate (eg, beta blockers), with instructions about activity level and clinician follow-up. (Level of evidence: C)Observe patients with symptoms consistent with ACS but without objective evidence of myocardial ischemia (nonischemic initial ECG and normal cardiac troponin) in a chest pain unit or telemetry unit with serial ECGs and cardiac troponin at 3- to 6-hour intervals. (Level of evidence: B)For patients with suspected ACS who have normal serial ECGs and cardiac troponin levels, it is reasonable to have obtain a treadmill ECG (Level of evidence: A), stress myocardial perfusion imaging, or stress echocardiography before discharge or within 72 hours after discharge. (Level of evidence: B)In patients with suspected ACS but a normal ECG, normal cardiac troponin levels, and no history of coronary artery disease (CAD), it is reasonable to initially perform (without serial ECGs and troponins) coronary computed tomography angiography to assess the coronary artery anatomy (Level of evidence: A) or rest myocardial perfusion imaging with a technetium-99m radiopharmaceutical to exclude myocardial ischemia. (Level of evidence: B)
The 2015 European Society of Cardiology (ESC) guidelines are in general agreement with the 2014 AHA/ACC guidelines. [1] Additional Class I recommendations are summarized below:
Base the diagnosis and initial short-term ischemic and bleeding risk stratification on a combination of the clinical history, symptoms, vital signs, other physical findings, and ECG and laboratory results. (Level of evidence: A)Measure cardiac troponin levels with sensitive or high-sensitivity assays, and obtain the results within 60 minutes of presentation. (Level of evidence: A)Perform a rapid rule-out protocol at 0 h and 3 h if high-sensitivity cardiac troponin tests are available. (Level of evidence: B)Perform a rapid rule-out and rule-in protocol at 0 h and 1 h if a high-sensitivity cardiac troponin test with a validated 0 h/1 h algorithm is available. Additional testing after 3–6 h is indicated if the first two troponin measurements are not conclusive and the patient's clinical condition remains suggestive of ACS. (Level of evidence: B)Perform continuous rhythm monitoring until the diagnosis of non–ST-elevation myocardial infarction (NSTEMI) is established or ruled out.(Level of evidence: C)In the absence of signs or symptoms of ongoing ischemia, rhythm monitoring in unstable angina may be considered in selected patients (eg, suspicion of coronary spasm or associated symptoms suggestive of arrhythmic events).
In addition, the ESC guidelines find the Global Registry of Acute Coronary Events (GRACE 2.0) risk calculation provides the most accurate stratification of risk both on admission and at discharge. [1] However, the guidelines caution that although its value as a prognostic assessment tool is clear, the impact of risk score implementation on patient outcomes has not been adequately investigated. Bleeding risk can be stratified using the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) risk score. [1]
Important considerations from the 2017 ESC guidelines for managing acute myocardial infarction in patients presenting with ST-segment elevation are summarized below. [108, 109]
Where fibrinolysis is the reperfusion strategy, the maximum time delay from the diagnosis of STEMI to treatment has been shortened from 30 minutes in 2012 to 10 minutes in 2017.
Complete revascularization in patients with multivessel disease receives a stronger recommendation, moving from class III (should not be performed) to class IIa (should be considered), with non–infarct-related arteries treated during the index procedure or another time point before discharge from the hospital.
Thrombus aspiration is no longer recommended, based on two large trials in more than 15,000 patients.
Deferred stenting, which involved opening the artery and waiting 48 hours to implant a stent, is no longer recommended.
For PCI, the use of drug-eluting stents instead of bare-metal stents has been upgraded from class IIa (should be considered) to class I (is recommended/indicated), as has the use of radial, instead of femoral, arterial access.
Dual antiplatelet therapy beyond 12 months may be considered in selected patients. Bivalirudin has been downgraded from class I to IIa (should be considered), and enoxaparin upgraded from class IIb (may be considered) to IIa (should be considered). Cangrelor (Kengreal), which was not mentioned in the 2012 document, has been recommended as an option in certain patients.
Additional lipid-lowering therapy is recommended in patients with high cholesterol despite taking the maximum dose of statins.
The cutoff for administering oxygen therapy has been lowered from less than 95% to less than 90% arterial oxygen saturation.
Left and right bundle branch block are now considered equal for recommending urgent angiography when patients have ischemic symptoms.
Selection of management strategy
Determination of the preferred management strategy depends on the patient’s clinical characteristics and clinical risk. The AHA/ACC and ESC provide similar recommendations for selection of the preferred management stategy, which are summarized in Table 3, below. [1, 110]
Table 3. Recommendations for Selection of Preferred Management Strategy (Open Table in a new window)
Preferred StrategyPatient Characteristic/Clinical Risk
Immediate invasive strategy
(< 2 hours)
Refractory anginaSigns or symptoms of heart failure, or new or worsening mitral regurgitationHemodynamic instability or cardiogenic shockRecurrent angina/ischemia at rest, or with low-level activities despite intensive medical therapySustained ventricular tachycardia or ventricular fibrillationIschemia-guided strategyLow-risk score (eg, TIMI 0 or 1, GRACE < 109)Low-risk Tn-negative femalePatient or physician preference in the absence of high-risk features
Early invasive strategy
(< 24 hours)
GRACE score >140Rise or fall in Tn compatible with MINew or presumably new ST-segment depression
Delayed invasive strategy
(24-72 hours)
Diabetes mellitusRenal insufficiency (GFR < 60 mL/min/1.73m2)Reduced LV systolic function (LVEF < 40%)Early postinfarction anginaPCI within 6 monthsPrior CABGGRACE score 109-140; TIMI Score ≥2ACC/AHA = American College of Cardiology/American Heart Association; CABG = coronary artery bypass grafting; GRACE = Global Registry of Acute Coronary Events; LV = left ventricle; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; TIMI = Thrombolysis in Myocardial Infarction Clinical Trial; Tn = troponin.
Initial hospital care
The 2014 AHA/ACC recommendations for initial hospital care are summarized below. [110]
Oxygen
Administer supplemental oxygen only when the oxygen saturation falls below 90%, respiratory distress is present, or other high-risk features for hypoxemia are present. (Class I; level of evidence C)
Nitrates
Administer sublingual nitroglycerin (NTG) every 5 minutes up to 3 times for continuing ischemic pain, and then assess the need for intravenous (IV) NTG. (Class I; level of evidence: C)
Administer IV NTG for persistent ischemia, heart failure (HF), or hypertension. (Class I; level of evidence: B)
Nitrates are contraindicated with recent use of a phosphodiesterase inhibitor. (Class III; level of evidence: B)
Analgesics
IV morphine sulfate may be reasonable for continued ischemic chest pain despite maximally tolerated anti-ischemic medications. (Class IIb; level of evidence: B)
Nonsteroidal anti-inflammatories (NSAIDs) (except aspirin) should not be initiated and should be discontinued because of the increased risk of major adverse cardiac events (MACE) associated with their use. (Class III; level of evidence: B)
Beta-adrenergic blockers
Initiate oral beta blockers in the absence of HF, low-output state, risk for cardiogenic shock, or other contraindications to beta blockade. (Class I; level of evidence: A)
Use sustained-release metoprolol succinate, carvedilol, or bisoprolol for beta-blocker therapy in patients with concomitant NSTE-ACS, stabilized HF, and reduced systolic function (Class I; level of evidence: C)
Re-evaluate to determine subsequent eligibility in patients with initial contraindications to beta blockers. (Class I; level of evidence: C)
It is reasonable to continue beta-blocker therapy in patients with normal LV function with NSTE-ACS. (Class IIa; level of evidence: C)
IV beta blockers are potentially harmful when risk factors for shock are present. (Class III; level of evidence: B)
Calcium channel blockers (CCBs)
Administer initial therapy with nondihydropyridine CCBs in patients with recurrent ischemia and contraindications to beta blockers in the absence of LV dysfunction, increased risk for cardiogenic shock, PR interval longer than 0.24 s, or second- or third-degree atrioventricular block without a cardiac pacemaker. (Class I; level of evidence: B)
Administer oral nondihydropyridine calcium antagonists in patients with recurrent ischemia after use of beta blockers and nitrates in the absence of contraindications. (Class I; level of evidence: C)
CCBs are recommended for ischemic symptoms when beta blockers are not successful, are contraindicated, or cause unacceptable side effects. (Class I; level of evidence: C)
Long-acting CCBs and nitrates are recommended for patients with coronary artery spasm. (Class I; level of evidence: C)
Immediate-release nifedipine is contraindicated in patients with NSTE-ACS in the the absence of a beta blocker therapy (Class III; level of evidence: B)
Cholesterol management
Initiate or continue high-intensity statin therapy in patients with no contraindications. (Class I; level of evidence: A)
Obtain a fasting lipid profile in patients with NSTE-ACS, preferably within 24 hours of presentation. (Class IIa; level of evidence:C)
Angiotensin-converting enzyme (ACE) inhibitors
Class I
ACE inhibitors should be started and continued indefinitely in all patients with a left ventricular ejection fraction (LVEF) below 40% and in those with hypertension, diabetes mellitus, or stable chronic kidney disease (CKD), unless contraindicated. (Level of evidence: A)
Use angiotensin receptor blockers (ARBs) in patients with heart failure or MI with an LVEF below 40% who are ACE inhibitor intolerant. (Level of evidence: A)
Use aldosterone blockade in post–MI patients who are without significant renal dysfunction or hyperkalemia who are receiving therapeutic doses of ACE inhibitor and beta blockers and have an LVEF below 40%, diabetes mellitus, or heart failure. (Level of evidence: A)
Antiplatelet therapy
The 2014 AHA/ACC recommendations for initial antiplatelet/anticoagulation therapy in patients with NSTE-ACS are summarized below. [110]
Aspirin
Non–enteric-coated, chewable aspirin (162 mg to 325 mg) should be given to all patients without contraindications as soon as possible after presentation, and a maintenance dose of aspirin (81 mg/d to 325 mg/d) should be continued indefinitely. (Class I; level of evidence A)
In patients who are unable to take aspirin because of hypersensitivity or major gastrointestinal (GI) intolerance, administer a loading dose of clopidogrel followed by a daily maintenance dose. (Class I; level of evidence B)
Anticoagulation
Administer anticoagulation, in addition to antiplatelet therapy, for all patients, irrespective of the initial treatment strategy. Treatment options include the following (all Class I):
Subcutaneous (SC) enoxaparin for the duration of hospitalization or until PCI is performed (Level of evidence: A)Bivalirudin until diagnostic angiography or PCI is performed in patients with early invasive strategy only (Level of evidence: B)SC fondaparinux for the duration of hospitalization or until PCI is performed (Level of evidence: B)IV unfractionated heparin (UFH) for 48 h or until PCI is performed (Level of evidence: B)
IV fibrinolytic treatment is not recommended in patients with NSTE-ACS. (Class III, level of evidence: A)
The 2017 ESC focused update recommendations for antiplatelet therapy in coronary artery disease are outlined below. [112, 113]
Predicting perioperative bleeding
Preoperative fibrinogen levels may be considered to identify patients at high risk of bleeding.
Routine use of viscoelastic and platelet function testing is not recommended to predict bleeding in patients without antithrombotic treatment.
Platelet function testing may be considered to guide the decision on the timing of cardiac surgery in patients who have recently received P2Y12 inhibitors or who have ongoing dual antiplatelet therapy (DAPT).
Managing preoperative anticoagulants and antiplatelet drugs
In patients undergoing coronary artery bypass grafting (CABG), acetylsalicylic acid (ASA) should be continued throughout the preoperative period.
In patients at high risk of bleeding or refusing blood transfusions and undergoing non-coronary cardiac surgery, stopping ASA should be considered at least 5 days preoperatively.
It is recommended that ASA be re(started) as soon as there is no concern over bleeding (within 24 hr) after isolated CABG.
In patients taking DAPT who need to have non-emergent cardiac surgery, postponing surgery for at least 3 days after discontinuation of ticagrelor, 5 days after clopidogrel, and 7 days after prasugrel should be considered.
It is recommended that GPIIb/IIIa inhibitors be discontinued at least 4 hours before surgery.
It is recommended that prophylactic low-molecular-weight heparin (LMWH) be discontinued 12 hours before surgery and fondaparinux 24 hours before surgery. A longer interval may be necessary for patients with impaired renal function and/or therapeutic doses.
Elective cardiac surgery should be performed if the international normalized ratio (INR) is < 1.5 in patients taking vitamin K antagonists (VKAs). When surgery cannot be postponed, coagulation factors should be used to reverse the effect.
In patients having elective cardiac surgery, direct oral anticoagulants (DOACs) should be stopped at least 48 hours before surgery. A longer interval may be necessary for patients with impaired renal function.
Preoperative anemia
Oral or intravenous iron alone prior to cardiac surgery may be considered in mildly anemic patients (women, hemoglobin (Hb) 100–120 g/L; men, Hb 100–130 g/L) or in severely anemic patients (both genders, Hb ≤100 g/L) to improve erythropoiesis.
Erythropoietin with iron supplementation should be considered to reduce postoperative transfusions in patients with non-iron deficiency (eg, erythropoietin (EPO), vitamin D, or folate acid deficiency) undergoing elective surgery.
Intraoperative anticoagulation
Heparin-level-guided heparin management should be considered over activated clotting time (ACT)-guided heparin management to reduce bleeding.
Heparin-level-guided protamine dosing may be considered to reduce bleeding and transfusions.
Protamine should be administered in a protamine-to-heparin dosing ratio < 1:1 to reduce bleeding.
Antithrombin (AT) supplementation is indicated in patients with AT deficiency to improve heparin sensitivity.
In patients with heparin-induced thrombocytopenia (HIT) antibodies for whom surgery cannot be postponed, anticoagulation with bivalirudin should be considered when the bleeding risk is acceptable. The use of heparin in the pre- and postoperative periods should be avoided.
Transfusion strategies
Implementation of a patient blood management protocol for the bleeding patient is recommended.
The use of packed red blood cells (PRBCs) of all ages is recommended, because the storage time of the PRBCs does not affect the outcomes.
The use of leukocyte-depleted PRBCs is recommended to reduce infectious complications.
Pooled solvent detergent fresh-frozen plasma (FFP) may be preferred to standard FFP to reduce the risk of transfusion-related acute lung injury.
Perioperative treatment algorithms for the bleeding patient based on viscoelastic point-of-care tests should be considered to reduce the number of transfusions.
Platelet concentrate should be transfused in bleeding patients with a platelet count below 50 (109/L) or patients on antiplatelet therapy with bleeding complications.
Dual antiplatelet therapy
In 2016, the ACC/AHA released updated guidelines on duration of dual antiplatelet therapy (DAPT) in patients with coronary artery disease. In this focused update, the term and acronym DAPT is used to specifically to refer to combination antiplatelet therapy with aspirin and a P2Y12 receptor inhibitor (clopidogrel, prasugrel, or ticagrelor).
Key recommendations for patients with NSTE-ACS or STEMI treated with DAPT are summarized below. [114]
Class I
For all patients treated with DAPT, a daily dose of aspirin 81 mg (range, 75 mg to 100 mg). (Level of evidence: B-R)
After implantation with a bare metal stent (BMS) or drug-eluting stent (DES), administer P2Y12 inhibitor therapy (clopidogrel, prasugrel, or ticagrelor) for at least 12 months. (Level of evidence: B-R)
For patients who subsequently undergo coronary artery bypass grafting (CABG) after coronary stent implantation, resume P2Y12 inhibitor therapy postoperatively so that DAPT continues until the recommended duration of therapy is completed. (Level of evidence: C-EO)
In patients who undergo CABG, resume P2Y12 inhibitor therapy after CABG to complete 12 months of DAPT therapy. (Level of evidence: C-LD)
Patients with STEMI treated with fibrinolytic therapy should be continue P2Y12 inhibitor therapy (clopidogrel) for a minimum of 14 days (Level of evidence: A) and, ideally, at least 12 months. (Level of evidence: C-EO)
Class IIa
After coronary stent implantation, it is reasonable to use ticagrelor in preference to clopidogrel for maintenance P2Y12 inhibitor therapy. (Level of evidence: B-R)
After coronary stent implantation in patients who are not at high risk for bleeding complications and who do not have a history of stroke or transient ischemic attack (TIA), it is reasonable to choose prasugrel over clopidogrel for maintenance P2Y12 inhibitor therapy. (Level of evidence: B-R)
Class IIb
In patients treated with coronary stent implantation or fibrinolytic therapy who have tolerated DAPT without a bleeding complication and who are not at high bleeding risk, it may be reasonable to continue DAPT (clopidogrel, prasugrel, or ticagrelor) for longer than 12 months. (Level of evidence: A)
After DES implantation, patients who develop a high risk of bleeding, are at high risk of severe bleeding complications, or develop significant overt bleeding, it may be reasonable to discontinue P2Y12 inhibitor therapy after 6 months. (Level of evidence: C-LD)
Class III
Prasugrel should not be administered to patients with a prior history of stroke or TIA. (Level of evidence: B-R)
Concomitant use of proton pump inhibitors and thienopyridines
A 2010 consensus statement issued by the ACC, American College of Gastroenterology (ACG), and AHA addressed the issue of concomitant use of proton pump inhibitors (PPIs) and thienopyridine antiplatelet drugs. [115] The recommendations are summarized below.
Clopidogrel reduces major cardiovascular (CV) events compared with placebo or aspirin.
Dual antiplatelet therapy with clopidogrel and aspirin, compared with aspirin alone, reduces major cardiovascular events and coronary stent thrombosis, but it is not routinely recommended for patients with prior ischemic stroke because of the risk of bleeding.
Clopidogrel alone, aspirin alone, and their combination are all associated with increased risk of GI bleeding.
Patients with prior GI bleeding are at highest risk for recurrent bleeding on antiplatelet therapy. Other clinical characteristics that increase the risk of GI bleeding include advanced age; concurrent use of anticoagulants, steroids, or NSAIDs including aspirin; and Helicobacter pylori infection. The risk of GI bleeding increases as the number of risk factors increases.
Use of a PPI or histamine H2 receptor antagonist (H2RA) reduces the risk of upper GI bleeding compared with no therapy. PPIs reduce upper GI bleeding to a greater degree than do H2RAs.
PPIs are recommended to reduce GI bleeding among patients with a history of upper GI bleeding. PPIs are appropriate in patients with multiple risk factors for GI bleeding who require antiplatelet therapy.
Routine use of either a PPI or an H2RA is not recommended for patients at a lower risk of upper GI bleeding.
Clinical decisions regarding concomitant use of PPIs and thienopyridines must balance overall risks and benefits, considering both cardiovascular and GI complications.
Dyslipidemia
In August 2019, the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) released updates to their 2016 guidelines for the management of dyslipidemia. [116, 117] Among the changes are new and more aggressive proposed goals for low-density lipoprotein cholesterol (LDL-C) levels, revised cardiovascular (CV) risk stratification, particularly for patients at high to very high risk, as well as new patient management recommendations. [116, 117]
New LDL targets across CV risk categories are as follows:
For very-high-risk patients (10-year risk of CV death ≥10%): Use an LDL-C reduction of at least 50% from baseline and an LDL-C goal of below 1.4 mmol/L (< 55 mg/dL).For very high-risk patients who experience a second vascular event within 2 years (not necessarily of the same type as the first event) while taking maximally tolerated statin therapy: An LDL-C goal of below 1.0 mmol/L (< 40 mg/dL) may be considered.For patients at high risk (10-year risk for CV death of 5% to < 10%): Use an LDL-C reduction of at least 50% from baseline and an LDL-C goal of below 1.8 mmol/L (< 70 mg/dL).For individuals at moderate risk (10-year risk for CV death of 1% to < 5%): Consider an LDL-C goal of below 2.6 mmol/L (< 100 mg/dL).For individuals at low risk (10-year risk for CV death < 1%): Consider an LDL-C goal of below 3.0 mmol/L (< 116 mg/dL).
New Recommendations
Cardiovascular imaging for assessment of ASCVD risk (should be considered), as follows:
Consider assessment of carotid and/or femoral arterial plaque burden on arterial ultrasonography as a risk modifier in individuals at low or moderate risk.Consider coronary artery calcium (CAC) score assessment with computed tomography (CT) as a risk modifier in the CV risk assessment of asymptomatic individuals at low or moderate risk.
Lipid analyses for CVD risk estimation (should be considered): Consider measurement of lipoprotein(a) (Lp(a)) at least once in each adult’s lifetime to identify those with very high inherited Lp(a) levels above 180 mg/dL (>430 nmol/L) who may have a lifetime risk of atherosclerotic CV disease (ASCVD) that is equivalent to the risk associated with heterozygous familial hypercholesterolemia (FH).
Pharmacotherapy of patients with hypertriglyceridemia (should be considered): In high-risk (or above) patients with triglyceride (TG) levels between 1.5 and 5.6 mmol/L (135-499 mg/dL) despite statin treatment, consider the combination of n-3 polyunsaturated fatty acids (PUFAs) (icosapent ethyl 2 × 2g/day) with statins.
Treatment of patients with heterozygous FH (should be considered): For primary prevention in individuals with FH at very-high risk, consider an LDL-C reduction of over 50% from baseline and an LDL-C goal below 1.4 mmol/L (< 55 mg/dL).
Recommendations for the treatment of dyslipidaemias in older people (aged >65 years) include the following:
For primary prevention in older people aged up to 75 years, statin therapy is recommended based on the level of risk.For primary prevention in older people older than 75 years, initiation of statin treatment may be considered if they are at high risk or above.
Dyslipidemia therapy in the setting of diabetes mellitus inlcudes the following:
For patients with type 2 diabetes mellitus (T2DM) at very-high risk, an LDL-C reduction of at least 50% from baseline and an LDL-C goal of below 1.4 mmol/L (< 55mg/dL) is recommended.For those with T2DM at high risk, an LDL-C reduction of at least 50% from baseline and an LDL-C goal of below 1.8 mmol/L (< 70 mg/dL) is recommended.For individuals with T1DM who are at high or very-high risk, statins are recommended.Consider intensification of statin therapy before introducing combination therapy. If the goal is not reached, consider a statin combined with ezetimibe. (Each should be considered.)Statin therapy is not recommended in premenopausal diabetic patients who are considering pregnancy or who are not using adequate contraception.
Lipid-lowering therapy in patients with ACS (should be considered): For patients who present with an acute coronary syndrome (ACS), and whose LDL-C levels are not at goal despite already taking a maximally tolerated statin dose and ezetimibe, consider adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor early after the event (if possible, during hospitalization for the ACS event).
2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction
In 2015 the ACC, AHA, and Society for Cardiovascular Angiography and Interventions (SCAI) issued revised recommendations for PCI in STEMI patients, summarized below. [118]
Class IIb
PCI should not be performed in a noninfarct artery at the time of primary PCI in patients who are hemodynamically stable. (Level of evidence: B)
PCI of a noninfarct artery may be considered in selected patients with multivessel disease who are hemodynamically stable, either at the time of primary PCI or as a planned staged procedure. (Level of evidence: B-R)
The usefulness of selective and bailout aspiration thrombectomy in patients undergoing primary PCI is not well established. (Level of evidence: C-LD)
Class III
Routine aspiration thrombectomy before primary PCI is not useful. (Level of evidence: A)
Hospital discharge and follow-up
Selected Class 1 recommendations for post-hospital care from the 2014 AHA/ACC guidelines are summarized below. [110]
Inpatient medications to control ischemia should be continued after hospital discharge in patients with NSTE-ACS who do not undergo coronary revascularization, patients with incomplete or unsuccessful revascularization, and patients with recurrent symptoms after revascularization. Titration of the doses may be required. (Level of evidence: C)
All patients should be given sublingual or spray NTG with verbal and written instructions for its use. (Level of evidence: C)
Before hospital discharge, patients should be informed about symptoms of worsening myocardial ischemia and Ml, and they should be given verbal and written instructions about how and when to seek emergency care for such symptoms. (Level of evidence: C)
For post–NSTE-ACS patients who have initial angina lasting more than 1 minute, it is recommended that NTG (1 dose sublingual or spray) be taken if angina does not subside within 3 to 5 minutes; patients or bystanders should call 9-1-1 immediately to access emergency medical services. (Level of evidence: C)
If the pattern or severity of angina changes, suggesting worsening myocardial ischemia (eg, pain is more frequent or severe or is precipitated by less effort or occurs at rest), patients should contact their clinician without delay to assess the need for additional treatment or testing. (Level of evidence: C)
Before discharge, patients should be educated about modification of cardiovascular risk factors. (Level of evidence: C)
Medication Summary
The goals of treatment are to preserve patency of the coronary artery, augment blood flow through stenotic lesions, and reduce myocardial oxygen demand. All patients should receive antiplatelet agents, and patients with evidence of ongoing ischemia should receive aggressive medical intervention until signs of ischemia, as determined by symptoms and ECG, resolve.
Antiplatelet agents
Class Summary
Antiplatelets inhibit the cyclooxygenase system, decreasing the level of thromboxane A2, which is a potent platelet activator. Antiplatelet therapy reduces mortality by reducing the risk of fatal strokes and fatal myocardial infarctions.
Early administration of aspirin (eg, Anacin, Ascriptin, Bayer Aspirin) in patients with acute myocardial infarction may reduce cardiac mortality in the first month. The adult dose is 160-324 mg PO or chewed. It can be administered as a suppository if the patient is unable to take PO medications. Aspirin reduces morbidity and mortality and is continued indefinitely. If administered with ticagrelor (Brilinta), do not exceed 100 mg/day after a one-time loading dose of 325 mg.
Vorapaxar reversibly inhibits protease-activated receptor 1 (PAR-1) which is expressed on platelets, but its long half-life makes it effectively irreversible. It is indicated to reduce thrombotic cardiovascular events in patients with a history of MI or with peripheral arterial disease. It is not used as monotherapy, but added to aspirin and/or clopidogrel.
Nitrates
Class Summary
Nitrates oppose coronary artery spasm and reduce myocardial oxygen demand by reducing preload and afterload.
Nitroglycerin (Nitro-Bid) causes relaxation of the vascular smooth muscle via stimulation of intracellular cyclic guanosine monophosphate production, causing a decrease in blood pressure. Nitrates do not improve mortality. However, they provide symptomatic relief by means of several mechanisms, including coronary vasodilation, improved collateral blood flow, decrease in preload (venodilation and reduced venous return), and decrease in afterload (arterial vasodilation). Care should be taken to avoid hypotension, because this can potentially reduce coronary perfusion pressure (diastolic BP - LV diastolic pressure).
Analgesics
Class Summary
These agents reduce pain which decreases sympathetic stress, in addition to providing some preload reduction.
Morphine sulfate (Duramorph, Astramorph, MS Contin) is the drug of choice for narcotic analgesia because of its reliable and predictable effects, safety profile, and ease of reversibility with naloxone. Morphine sulfate administered intravenously may be dosed in a number of ways and commonly titrated until the desired effect is obtained.
Beta-adrenergic blockers
Class Summary
Beta blockers have antiarrhythmic and antihypertensive properties, as well as the ability to reduce ischemia. They minimize the imbalance between myocardial supply and demand by reducing afterload and wall stress. In patients with acute MI, they decrease infarct size as well as short- and long-term mortality, which is a function of their anti-ischemic and antiarrhythmic properties. These drugs may prevent mechanical complications of myocardial infarction, including rupture of the papillary muscle, left ventricular free wall, and ventricular septum. Beta blockers ameliorate dynamic obstruction of the left ventricular outflow tract in patients with apical infarct and hyperdynamic basal segments. They should not be used acutely in patients with cardiogenic shock or signs of heart failure on presentation.
Metoprolol (Lopressor)
Metoprolol (Lopressor) is a selective beta1-adrenergic receptor blocker that decreases the automaticity of contractions. During IV administration, blood pressure, heart rate, and ECG should be carefully monitored. The goal of treatment is to reduce the patient's heart rate to 60-90 beats/min.
Esmolol (Brevibloc) is an excellent drug for use in patients at risk for complications from beta blockers, particularly reactive airway disease, mild to moderate LV dysfunction, and peripheral vascular disease. Its short half-life of 8 min allows for titration to desired effect with the ability to stop quickly prn.
Glycoprotein IIB/IIIA inhibitors
Class Summary
Glycoprotein IIb/IIIa receptor antagonists include abciximab, eptifibatide, and tirofiban. Glycoprotein IIb/IIIa antagonists prevent the binding of fibrinogen, thereby blocking platelet aggregation. These drugs inhibit the glycoprotein IIb/IIIa receptor, which is involved in the final common pathway for platelet adhesion and aggregation. Currently, GP IIb/IIIb receptor antagonists in combination with aspirin are considered standard antiplatelet therapy for patients at high risk for unstable angina.
Abciximab (ReoPro) is a chimeric human-murine monoclonal antibody. It binds to receptors with high affinity and reduces platelet aggregation by 80%. Inhibition of platelet aggregation persists for up to 48 hours after the end of infusion. Abciximab has been approved for use in elective/urgent/emergent percutaneous coronary intervention.
Eptifibatide (Integrilin) is an antagonist of the platelet GP IIb/IIIa receptor; it reversibly prevents von Willebrand factor, fibrinogen, and other adhesion ligands from binding to the GP IIb/IIIa receptor. The end effect is the inhibition of platelet aggregation. The effects persist over the duration of maintenance infusion and are reversed when infusion ends. Use eptifibatide (or tirofiban, see below) in patients with high-risk features in whom invasive treatment is not planned.
Tirofiban (Aggrastat) is a nonpeptide antagonist of the GP IIb/IIIa receptor. It is a reversible antagonist of fibrinogen binding. When administered intravenously, more than 90% of platelet aggregation is inhibited. Tirofiban has been approved to reduce the rate of thrombotic cardiovascular events (combined endpoint of death, myocardial infarction, or refractory ischemia/repeat cardiac procedure) in patients with non-ST elevation acute coronary syndrome (NSTE-ACS).
Anticoagulants
Class Summary
Anticoagulants are used to prevent recurrence of clot after a spontaneous fibrinolysis.
Heparin augments the activity of antithrombin III and prevents the conversion of fibrinogen to fibrin. It does not actively lyse but is able to inhibit further thrombogenesis. This agent prevents recurrence of a clot after spontaneous fibrinolysis.
Low molecular weight heparins
Class Summary
LMWH is indicated for treatment of ST-segment elevation myocardial infarction (STEMI) managed medically or with subsequent PCI. It is also indicated as prophylaxis for ischemic complications caused by unstable angina and non–Q-wave myocardial infarction.
Aside from the possible medical benefits of using LMWH in place of unfractionated heparin, advantages of LMWH include ease of administration, absence of need for anticoagulation monitoring, and potential for overall cost savings. Although 3 LMWHs are approved for use in the United States, only enoxaparin is currently approved for use in unstable angina.
Low-molecular-weight heparin (enoxaparin; Lovenox), which is produced by partial chemical or enzymatic depolymerization of unfractionated heparin, binds to antithrombin III, enhancing its therapeutic effect. The heparin–antithrombin III complex binds to and inactivates activated factor X (Xa) and factor II (thrombin). LMWH differs from unfractionated heparin by having a higher ratio of antifactor Xa to antifactor IIa than does unfractionated heparin. Maximum antifactor Xa and antithrombin activities occur 3-5 hours after administration.
Direct thrombin inhibitors
Class Summary
Direct thrombin inhibitors bind directly to the anion binding site and the catalytic sites of thrombin to produce potent and predictable anticoagulation.
Hirudin (Lepirudin, Refludan) is the prototype of direct thrombin inhibitors. Hirudin binds directly to the anion binding site and the catalytic sites of thrombin to produce potent and predictable anticoagulation. Currently, hirudin is indicated only in patients who are unable to receive heparin because of heparin-induced thrombocytopenia.
Bivalirudin (Angiomax) is a synthetic analogue of recombinant hirudin. It inhibits thrombin and is used for anticoagulation in unstable angina in patients undergoing PTCA. Potential advantages over conventional heparin therapy include more predictable and precise levels of anticoagulation, activity against clot-bound thrombin, absence of natural inhibitors (eg, platelet factor 4, heparinase), and continued efficacy following clearance from plasma (because of binding to thrombin).
Adenosine diphosphate receptor antagonists
Class Summary
Thienopyridine adenosine 5'-diphosphate (ADP) antagonists approved for antiplatelet activity in the United States include clopidogrel, ticlopidine, prasugrel, and ticagrelor. All but ticagrelor have irreversible antiplatelet activity and take several days to manifest an effect, whereas, ticagrelor is a reversible P2Y12 receptor inhibitor. A potential additive benefit exists when ADP antagonists are used in conjunction with aspirin. These drugs may be considered alternatives to aspirin in patients with aspirin intolerance or who are allergic to aspirin.
Clopidogrel (Plavix) inhibits ADP-dependent activation of the glycoprotein IIb/IIIa complex, a necessary step for platelet aggregation. This process results in intense inhibition of platelet function, particularly in combination with aspirin.
Clopidogrel can be considered an alternative to aspirin in patients with aspirin intolerance or who are allergic to aspirin. The CURRENT-OASIS 7 trial suggests that a 7-day double-dose clopidogrel regimen can be considered for patients with acute coronary syndromes, as the efficacy and safety did not differ from that of a high- and low-dose aspirin regimen. However, there is no benefit in the double-dose treatment for patients who are undergoing an early invasive strategy.
Clopidogrel is a class I recommendation for patients when an early noninterventional approach is planned in therapy. When percutaneous coronary intervention (PCI) is planned, clopidogrel is started and continued for at least 1 month and for up to 9 months, if the patient is not at high risk for bleeding.
Clopidogrel is generally preferred over ticlopidine (Ticlid), because it more rapidly inhibits platelets and appears to have a more favorable safety profile.
Clopidogrel has been suggested to be less effective in reducing the rate of cardiovascular events in individuals who carry the loss-of-function CYP2C19 alleles. However, a 2010 study concluded that patients with ACS or atrial fibrillation respond well to clopidogrel, regardless of CYP2C19 loss-of-function carrier status.
Beneficial effects were noted in patients with UA after 2 wk of use in one randomized trial. When compared to controls, ticlopidine use decreased vascular deaths and nonfatal MIs.
Thienopyridine drug that inhibits platelet activation and aggregation through the irreversible binding of its active metabolite to ADP platelet receptors (specifically the P2Y12 receptor). Platelet inhibition is the result of this action.
Indicated to reduce thrombotic cardiovascular (CV) events (including stent thrombosis) with acute coronary syndrome (ACS) that is managed with percutaneous coronary intervention (PCI). Specifically for unstable angina or non – ST-elevation myocardial infarction (NSTEMI) or with ST-elevation myocardial infarction (STEMI) when managed with primary or delayed PCI.
Reduces rate of combined endpoint of CV death, nonfatal MI, or nonfatal stroke compared with clopidogrel.
Ticagrelor and its major metabolite reversibly interact with the platelet P2Y12 ADP-receptor to prevent signal transduction and platelet activation. It is indicated to reduce the rate of thrombotic cardiovascular (CV) events in patients with ACS (unstable angina, non-ST elevation MI, or ST elevation MI). It also reduces the rate of stent thrombosis in patients who have undergone stent placement for treatment of ACS and is indicated in patients with a history of MI more than 1 year previously.
Clinical trial results showed a reduced rate of combined endpoint of CV death, MI, or stroke compared to clopidogrel. Difference between treatments was driven by CV death and MI with no difference in stroke. In patients treated with PCI, ticagrelor also reduces the rate of stent thrombosis.
The drug is administered with aspirin (loading dose of 325 mg PO once, then 75-100 mg/day). Note that exceeding an aspirin dose of 100 mg/day decreases ticagrelor effectiveness.
References
1 [Guideline] Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016 Jan 14. 37(3):267-315. [Medline]. [Full Text].
2 [Guideline] Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012 Oct 16. 126(16):2020-35. [Medline]. [Full Text].
Gardner LS, Nguyen-Pham S, Greenslade JH, et al. Admission glycaemia and its association with acute coronary syndrome in Emergency Department patients with chest pain. Emerg Med J. 2015 Aug. 32(8):608-12. [Medline].
3 Boggs W. Blood glucose predicts outcomes of patients with chest pain. Reuters Health Information. November 11, 2014. [Full Text].
4 Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996 Oct 31. 335(18):1342-9. [Medline]. [Full Text].
5 Heidenreich PA, Alloggiamento T, Melsop K, et al. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol. 2001 Aug. 38(2):478-85. [Medline]. [Full Text].
6 Bangalore S, Qin J, Sloan S, Murphy SA, Cannon CP. What is the optimal blood pressure in patients after acute coronary syndromes?: Relationship of blood pressure and cardiovascular events in the PRavastatin OR atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction (PROVE IT-TIMI) 22 trial. Circulation. 2010 Nov 23. 122(21):2142-51. [Medline].
7 LeLeiko RM, Vaccari CS, Sola S, et al. Usefulness of elevations in serum choline and free F2)-isoprostane to predict 30-day cardiovascular outcomes in patients with acute coronary syndrome. Am J Cardiol. 2009 Sep 1. 104(5):638-43. [Medline].
Ma RC, Tong PC. Testosterone levels and cardiovascular disease. Heart. 2010 Nov. 96(22):1787-8. [Medline].
8 Sanchis J, Nunez J, Bodi V, et al. Influence of comorbid conditions on one-year outcomes in non-ST-segment elevation acute coronary syndrome. Mayo Clin Proc. 2011 Apr. 86(4):291-6. [Medline]. [Full Text].
9 Gurm HS, Gore JM, Anderson FA Jr, et al, for the Global Registry of Acute Coronary Events (GRACE) Investigators. Comparison of acute coronary syndrome in patients receiving versus not receiving chronic dialysis (from the Global Registry of Acute Coronary Events [GRACE] Registry). Am J Cardiol. 2012 Jan 1. 109(1):19-25. [Medline].
Chughtai H, Ratner D, Pozo M, et al. Prehospital delay and its impact on time to treatment in ST-elevation myocardial infarction. Am J Emerg Med. 2011 May. 29(4):396-400. [Medline].
10 Wood S. STEMI in Women: Same Plaques, Same Stent Outcomes: OCTAVIA. Medscape Medical News. Available at http://www.medscape.com/viewarticle/825391. Accessed: May 27, 2014.
11 Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011 Mar 26. 377(9771):1077-84. [Medline].
12 [Guideline] Chou R, for the High Value Care Task Force of the American College of Physicians. Cardiac screening with electrocardiography, stress echocardiography, or myocardial perfusion imaging: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015 Mar 17. 162(6):438-47. [Medline]. [Full Text].
13 Scheuermeyer FX, Innes G, Grafstein E, et al. Safety and efficiency of a chest pain diagnostic algorithm with selective outpatient stress testing for emergency department patients with potential ischemic chest pain. Ann Emerg Med. 2012 Apr. 59(4):256-264.e3. [Medline].
14 Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011 Dec 28. 306(24):2684-93. [Medline].
15 O'Neil BJ, Hoekstra J, Pride YB, et al. Incremental benefit of 80-lead electrocardiogram body surface mapping over the 12-lead electrocardiogram in the detection of acute coronary syndromes in patients without ST-elevation myocardial infarction: Results from the Optimal Cardiovascular Diagnostic Evaluation Enabling Faster Treatment of Myocardial Infarction (OCCULT MI) trial. Acad Emerg Med. 2010 Sep. 17(9):932-9. [Medline]. [Full Text].
16 Damman P, Holmvang L, Tijssen JG, et al. Usefulness of the admission electrocardiogram to predict long-term outcomes after non-ST-elevation acute coronary syndrome (from the FRISC II, ICTUS, and RITA-3 [FIR] Trials). Am J Cardiol. 2012 Jan 1. 109(1):6-12. [Medline].
17 Damman P, Wallentin L, Fox KA, et al. Long-term cardiovascular mortality after procedure-related or spontaneous myocardial infarction in patients with non-ST-segment elevation acute coronary syndrome: a collaborative analysis of individual patient data from the FRISC II, ICTUS, and RITA-3 trials (FIR). Circulation. 2012 Jan 31. 125(4):568-76. [Medline]. [Full Text].
18 Iliou MC, Fumeron C, Benoit MO, et al. Prognostic value of cardiac markers in ESRD: Chronic Hemodialysis and New Cardiac Markers Evaluation (CHANCE) study. Am J Kidney Dis. 2003 Sep. 42(3):513-23. [Medline].
Ohman EM, Armstrong PW, Christenson RH, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med. 1996 Oct 31. 335(18):1333-41. [Medline]. [Full Text].
Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000 Oct 19. 343(16):1139-47. [Medline].
Newby LK, Christenson RH, Ohman EM, et al. Value of serial troponin T measures for early and late risk stratification in patients with acute coronary syndromes. The GUSTO-IIa Investigators. Circulation. 1998 Nov 3. 98(18):1853-9. [Medline]. [Full Text].
Lindahl B, Venge P, Wallentin L. Relation between troponin T and the risk of subsequent cardiac events in unstable coronary artery disease. The FRISC study group. Circulation. 1996 May 1. 93(9):1651-7. [Medline].
Stubbs P, Collinson P, Moseley D, Greenwood T, Noble M. Prognostic significance of admission troponin T concentrations in patients with myocardial infarction. Circulation. 1996 Sep 15. 94(6):1291-7. [Medline].
Apple FS, Parvin CA, Buechler KF, Christenson RH, Wu AH, Jaffe AS. Validation of the 99th percentile cutoff independent of assay imprecision (CV) for cardiac troponin monitoring for ruling out myocardial infarction. Clin Chem. 2005 Nov. 51(11):2198-200. [Medline]. [Full Text].
Eggers KM, Oldgren J, Nordenskjold A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J. 2004 Oct. 148(4):574-81. [Medline].
Macrae AR, Kavsak PA, Lustig V, et al. Assessing the requirement for the 6-hour interval between specimens in the American Heart Association Classification of Myocardial Infarction in Epidemiology and Clinical Research Studies. Clin Chem. 2006 May. 52(5):812-8. [Medline]. [Full Text].
Kavsak PA, MacRae AR, Newman AM, et al. Effects of contemporary troponin assay sensitivity on the utility of the early markers myoglobin and CKMB isoforms in evaluating patients with possible acute myocardial infarction. Clin Chim Acta. 2007 May 1. 380(1-2):213-6. [Medline].
9 Meune C, Balmelli C, Twerenbold R, et al. Patients with acute coronary syndrome and normal high-sensitivity troponin. Am J Med. 2011 Dec. 124(12):1151-7. [Medline].
Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation. 2008 Nov 18. 118(21):2200-6. [Medline].
10 Sorensen JT, Terkelsen CJ, Steengaard C, et al. Prehospital troponin T testing in the diagnosis and triage of patients with suspected acute myocardial infarction. Am J Cardiol. 2011 May 15. 107(10):1436-40. [Medline].
11 Venge P, Ohberg C, Flodin M, Lindahl B. Early and late outcome prediction of death in the emergency room setting by point-of-care and laboratory assays of cardiac troponin I. Am Heart J. 2010 Nov. 160(5):835-41. [Medline].
12 O'Riordan M. Negative troponin and copeptin tests rule out acute coronary syndrome. Heartwire from Medscape Medical News. September 3, 2013. Available at http://www.medscape.com/viewarticle/810373. Accessed: September 20, 2013.
Misra D, Leibowitz K, Gowda RM, Shapiro M, Khan IA. Role of N-acetylcysteine in prevention of contrast-induced nephropathy after cardiovascular procedures: a meta-analysis. Clin Cardiol. 2004 Nov. 27(11):607-10. [Medline].
13 Charpentier S, Cournot M, Lauque D, et al. Usefulness of initial glucose level to improve acute coronary syndrome diagnosis in the emergency department. Emerg Med J. 2011 Jul. 28(7):564-8. [Medline].
14 de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001 Oct 4. 345(14):1014-21. [Medline]. [Full Text].
15 James SK, Lindahl B, Siegbahn A, et al. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation. 2003 Jul 22. 108(3):275-81. [Medline]. [Full Text].
16 Hubbard BL, Newton CR, Carter PM, et al. The inability of B-type natriuretic protein to predict short-term risk of death or myocardial infarction in non-heart-failure patients with marginally increased troponin levels. Ann Emerg Med. 2010 Nov. 56(5):472-80. [Medline].
17 Cavusoglu E, Marmur JD, Hojjati MR, et al. Plasma interleukin-10 levels and adverse outcomes in acute coronary syndrome. Am J Med. 2011 Aug. 124(8):724-30. [Medline].
18 Giugliano RP, Braunwald E. The year in non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2008 Sep 23. 52(13):1095-103. [Medline].
19 Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006 Jul 4. 48(1):1-11. [Medline].
20 Hjortshoj S, Kristensen SR, Ravkilde J. Diagnostic value of ischemia-modified albumin in patients with suspected acute coronary syndrome. Am J Emerg Med. 2010 Feb. 28(2):170-6. [Medline].
21 Katritsis DG, Siontis GC, Kastrati A, et al. Optimal timing of coronary angiography and potential intervention in non-ST-elevation acute coronary syndromes. Eur Heart J. 2011 Jan. 32(1):32-40. [Medline].
22 Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000 Aug 16. 284(7):835-42. [Medline]. [Full Text].
23 Nabi F, Chang SM, Pratt CM, et al. Coronary artery calcium scoring in the emergency department: identifying which patients with chest pain can be safely discharged home. Ann Emerg Med. 2010 Sep. 56(3):220-9. [Medline].
24 Rogers IS, Banerji D, Siegel EL, et al. Usefulness of comprehensive cardiothoracic computed tomography in the evaluation of acute undifferentiated chest discomfort in the emergency department (CAPTURE). Am J Cardiol. 2011 Mar 1. 107(5):643-50. [Medline].
25 Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012 Apr 12. 366(15):1393-403. [Medline]. [Full Text].
26 S, Elashoff MR, Beineke P, et al, for the PREDICT (Personalized Risk Evaluation and Diagnosis in the Coronary Tree) Investigators. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med. 2010 Oct 5. 153(7):425-34. [Medline]. [Full Text].
17 CD, Hwang W, Hoekstra JW, et al. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Ann Emerg Med. 2010 Sep. 56(3):209-219.e2. [Medline]. [Full Text].
Rogers IS, Banerji D, Siegel EL, et al. Usefulness of comprehensive cardiothoracic computed tomography in the evaluation of acute undifferentiated chest discomfort in the emergency department (CAPTURE). Am J Cardiol. 2011 Mar 1. 107(5):643-50. [Medline].
18 SR, Yusuf S. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. 2000 Dec. 21(24):2033-41. [Medline].
29 GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011 Jan 20. 364(3):226-35. [Medline]. [Full Text].
30 GF, Kirtane AJ, Genereux P, et al. Impact of the presence and extent of incomplete angiographic revascularization after percutaneous coronary intervention in acute coronary syndromes: the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circulation. 2012 May 29. 125(21):2613-20. [Medline]. [Full Text].
31 Yan TD, Padang R, Poh C, et al. Drug-eluting stents versus coronary artery bypass grafting for the treatment of coronary artery disease: a meta-analysis of randomized and nonrandomized studies. J Thorac Cardiovasc Surg. 2011 May. 141(5):1134-44. [Medline].
32 Ribichini F, Tomai F, De Luca G, et al. Immunosuppressive therapy with oral prednisone to prevent restenosis after PCI. A multicenter randomized trial. Am J Med. 2011 May. 124(5):434-43. [Medline].
33 Stone GW, Witzenbichler B, Guagliumi G, et al. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet. 2011 Jun 25. 377(9784):2193-204. [Medline].
34 Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009 May 21. 360(21):2165-75. [Medline]. [Full Text].
35 Jernberg T, Johanson P, Held C, et al. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA. 2011 Apr 27. 305(16):1677-84. [Medline].
Thadani U, Opie LH. Nitrates for unstable angina. Cardiovasc Drugs Ther. 1994 Oct. 8(5):719-26. [Medline].
36 Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ. 1994 Jan 8. 308(6921):81-106. [Medline]. [Full Text].
37 Nijjer SS, Watson G, Athanasiou T, Malik IS. Safety of clopidogrel being continued until the time of coronary artery bypass grafting in patients with acute coronary syndrome: a meta-analysis of 34 studies. Eur Heart J. 2011 Dec. 32(23):2970-88. [Medline].
Morel O, El Ghannudi S, Jesel L, et al. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol. 2011 Jan 25. 57(4):399-408. [Medline]. [Full Text].
38 Dexilant (dexlansoprazole). Prescribing information revised October 28, 2011. Takeda Pharmaceutical Company LTD. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022287s011lbl.pdf.
39 Prevacid (lansoprazole). Prescribing information revised October 28, 2011. Takeda Pharmaceutical Company LTD. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020406s076,021428s023lbl.pdf.
40 Squizzato A, Keller T, Romualdi E, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database Syst Rev. 2011 Jan 19. CD005158. [Medline].
41 Simon T, Steg PG, Gilard M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. 2011 Feb 8. 123(5):474-82. [Medline]. [Full Text].
42 Morrow DA, Wiviott SD, White HD, et al. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction. Circulation. 2009 Jun 2. 119(21):2758-64. [Medline]. [Full Text].
43 Montalescot G, Collet JP, Ecollan P, et al, for the ACCOAST Investigators. Effect of prasugrel pre-treatment strategy in patients undergoing percutaneous coronary intervention for NSTEMI: the ACCOAST-PCI study. J Am Coll Cardiol. 2014 Dec 23. 64(24):2563-71. [Medline]. [Full Text].
44 Boggs W. Hold Prasugrel Until Revascularization Decision Is Made, Researchers Say. Medscape. Dec 18 2014. [Full Text].
Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007 Nov 15. 357(20):2001-15. [Medline]. [Full Text].
45 Roe MT, Armstrong PW, Fox KA, et al, for the TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012 Oct 4. 367(14):1297-309. [Medline]. [Full Text].
46 Scirica BM, Bonaca MP, Braunwald E, et al. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet. 2012 Oct 13. 380(9850):1317-24. [Medline].
47 James S, Akerblom A, Cannon CP, et al. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009 Apr. 157(4):599-605. [Medline].
48 Douglas D. Ticagrelor more cardioprotective than clopidogrel. Medscape Medical News. Jan 11, 2013. Available at http://www.medscape.com/viewarticle/777535. Accessed: January 23, 2013.
Kohli P, Wallentin L, Reyes E, et al. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO Study. Circulation. 2013 Feb 12. 127(6):673-80. [Medline]. [Full Text].
49 US FDA approves expanded indication for BRILINTA to include long-term use in patients with a history of heart attack [press release]. AstraZeneca. Available at http://www.astrazeneca.com/Media/Press-releases/Article/20150903. September 3, 2015; Accessed: September 9, 2015.
50 Bonaca MP, Bhatt DL, Cohen M, et al, for the PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015 May 7. 372(19):1791-800. [Medline]. [Full Text].
51 O'Riordan M. Ticagrelor bests clopidogrel for reducing stent-thrombosis risk: PLATO. Medscape Medical News. August 2, 2013. [Full Text].
52 Steg PG, Harrington RA, Emanuelsson H, et al, for the PLATO Study Group. Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial. Circulation. 2013 Sep 3. 128(10):1055-65. [Medline]. [Full Text].
53 Kastrati A, Mehilli J, Neumann FJ, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. 2006 Apr 5. 295(13):1531-8. [Medline]. [Full Text].
54 Roffi M, Moliterno DJ, Meier B, et al. Impact of different platelet glycoprotein IIb/IIIa receptor inhibitors among diabetic patients undergoing percutaneous coronary intervention: : Do Tirofiban and ReoPro Give Similar Efficacy Outcomes Trial (TARGET) 1-year follow-up. Circulation. 2002 Jun 11. 105(23):2730-6. [Medline]. [Full Text].
55 Roe MT, Harrington RA, Prosper DM, et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease.The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation. 2000 Sep 5. 102(10):1101-6. [Medline]. [Full Text].
56 Theroux P, Alexander J Jr, Pharand C, et al. Glycoprotein IIb/IIIa receptor blockade improves outcomes in diabetic patients presenting with unstable angina/non-ST-elevation myocardial infarction: results from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) study. Circulation. 2000 Nov 14. 102(20):2466-72. [Medline]. [Full Text].
57 Giugliano RP, White JA, Bode C, et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009 May 21. 360(21):2176-90. [Medline]. [Full Text].
58 Chadow HL, Hauptman RE, VanAuker M, et al. Drip and ship: a new strategy for the treatment of acute coronary syndromes. J Thromb Thrombolysis. 2000 Aug. 10(1):77-82. [Medline].
59 Januzzi JL, Chae CU, Sabatine MS, Jang IK. Elevation in serum troponin I predicts the benefit of tirofiban. J Thromb Thrombolysis. 2001 May. 11(3):211-5. [Medline].
Oler A, Whooley MA, Oler J, Grady D. Adding heparin to aspirin reduces the incidence of myocardial infarction and death in patients with unstable angina. A meta-analysis. JAMA. 1996 Sep 11. 276(10):811-5. [Medline].
60 Steg PG, Jolly SS, Mehta SR, et al. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010 Sep 22. 304(12):1339-49. [Medline]. [Full Text].
61 Fox KA, Antman EM, Cohen M, Bigonzi F. Comparison of enoxaparin versus unfractionated heparin in patients with unstable angina pectoris/non-ST-segment elevation acute myocardial infarction having subsequent percutaneous coronary intervention. Am J Cardiol. 2002 Sep 1. 90(5):477-82. [Medline].
62 Mahaffey KW, Ferguson JJ. Exploring the role of enoxaparin in the management of high-risk patients with non-ST-elevation acute coronary syndromes: the SYNERGY trial. Am Heart J. 2005 Apr. 149(4 Suppl):S81-90. [Medline].
63 Lev EI, Hasdai D, Scapa E, et al. Administration of eptifibatide to acute coronary syndrome patients receiving enoxaparin or unfractionated heparin: effect on platelet function and thrombus formation. J Am Coll Cardiol. 2004 Mar 17. 43(6):966-71. [Medline]. [Full Text].
64 Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011 Aug 25. 365(8):699-708. [Medline].
Boggs W. Lower rivaroxaban dose better in acute coronary syndrome. Medscape Medical News. May 30, 2013. [Full Text].
65 Mega JL, Braunwald E, Wiviott SD, et al. Comparison of the efficacy and safety of two rivaroxaban doses in acute coronary syndrome (from ATLAS ACS 2-TIMI 51). Am J Cardiol. 2013 Aug 15. 112(4):472-8. [Medline].
66 van Rees Vellinga TE, Peters RJ, Yusuf S, et al. Efficacy and safety of fondaparinux in patients with ST-segment elevation myocardial infarction across the age spectrum. Results from the Organization for the Assessment of Strategies for Ischemic Syndromes 6 (OASIS-6) trial. Am Heart J. 2010 Dec. 160(6):1049-55. [Medline].
67 Jolly SS, Faxon DP, Fox KA, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors or thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009 Jul 28. 54(5):468-76. [Medline]. [Full Text].
68 SS, Rao SV, Melloni C, et al, for the REVEAL Investigators. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011 May 11. 305(18):1863-72. [Medline]. [Full Text].
69 JT, Terkelsen CJ, Norgaard BL, et al. Urban and rural implementation of pre-hospital diagnosis and direct referral for primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction. Eur Heart J. 2011 Feb. 32(4):430-6. [Medline].
70 P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2010 Mar 2. 55(9):858-64. [Medline]. [Full Text].
71 GW, McLaurin BT, Cox DA, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006 Nov 23. 355(21):2203-16. [Medline]. [Full Text].
Kastrati A, Neumann FJ, Schulz S, et al. Abciximab and heparin versus bivalirudin for non-ST-elevation myocardial infarction. N Engl J Med. 2011 Nov 24. 365(21):1980-9. [Medline].
72 [Guideline] Knuuti J, Wijns W, Saraste A, et al, for the ESC Scientific Document Group . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2019 Aug 31. [Medline]. [Full Text].
73 S. 'Stable' CAD reconsidered in new ESC chronic coronary syndrome guideline. Medscape Medical News. September 1, 2019. Available at https://www.medscape.com/viewarticle/917552. Accessed: October 30, 2019.
74 Guideline] O'Gara PT, Kushner FG, Ascheim DD, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29. 127(4):e362-425. [Medline]. [Full Text].
75 [Guideline] Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012 Oct. 33(20):2569-619. [Medline]. [Full Text].
76 Guideline] Ibanez B, James S, Agewall S, et al, for the ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018 Jan 7. 39(2):119-77. [Medline].
77 Busco M. New ESC guideline on acute ST-segment elevation MI. Medscape Medical News. Available at https://www.medscape.com/viewarticle/885509. September 11, 2017; Accessed: August 27, 2018.
78 [Guideline] Amsterdam EA, Wenger NK, Brindis RG, et al, for the ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Dec 23. 130(25):e344-426. [Medline]. [Full Text].
79 [Guideline] Welsford M, Nikolaou NI, Beygui F, et al, for the Acute Coronary Syndrome Chapter Collaborators. Part 5: Acute coronary syndromes: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015 Oct 20. 132(16 Suppl 1):S146-76. [Medline]. [Full Text].
80 [Guideline] Valgimigli M, Bueno H, Byrne RA, et al, for the ESC Scientific Document Group, ESC Committee for Practice Guidelines (CPG), et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018 Jan 14. 39(3):213-60. [Medline].
81 Hughes S. ESC guideline update on dual antiplatelet therapy in CAD. Medscape Medical News. Available at https://www.medscape.com/viewarticle/885683. September 14, 2017; Accessed: August 27, 2018.
82 [Guideline] Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable isc... Circulation. 2016 Sep 6. 134(10):e123-55. [Medline]. [Full Text].
83 [Guideline] Abraham NS, Hlatky MA, Antman EM, et al, for the ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010 Dec 14. 122(24):2619-33. [Medline]. [Full Text].
84 [Guideline] Mach F, Baigent C, Catapano AL, et al, for the ESC Scientific Document Group . 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019 Aug 31. [Medline]. [Full Text].
85 Huges S. New European lipid guidelines take aggressive approach. Medscape Medical News. September 1, 2019. Available at https://www.medscape.com/viewarticle/917551. Accessed: October 3, 2019.
86 [Guideline] Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-Elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016 Mar 15. 133 (11):1135-47. [Medline]. [Full Text].
87 Bueno H, Betriu A, Heras M, et al. Primary angioplasty vs. fibrinolysis in very old patients with acute myocardial infarction: TRIANA (TRatamiento del Infarto Agudo de miocardio eN Ancianos) randomized trial and pooled analysis with previous studies. Eur Heart J. 2011 Jan. 32(1):51-60. [Medline]. [Full Text].
Chang AM, Walsh KM, Shofer FS, McCusker CM, Litt HI, Hollander JE. Relationship between cocaine use and coronary artery disease in patients with symptoms consistent with an acute coronary syndrome. Acad Emerg Med. 2011 Jan. 18(1):1-9. [Medline]. [Full Text].
88 James S, Angiolillo DJ, Cornel JH, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010 Dec. 31(24):3006-16. [Medline]. [Full Text].
89 Lopes RD, Alexander KP, Manoukian SV, et al. Advanced age, antithrombotic strategy, and bleeding in non-ST-segment elevation acute coronary syndromes: results from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll . 2009 Mar 24. 53(12):1021-30. [Medline]. [Full Text].
90 Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010 Nov. 96(22):1821-5. [Medline].
91 Metha SR, Tanguay JF, Eikelboom JW, et al, for the CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010 Oct 9. 376(9748):1233-43. [Medline].
92 Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al for the MERLIN-TIMI 36 Trial Investigators. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007 Apr 25. 297(16):1775-83. [Medline]. [Full Text].
93 Pare G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010 Oct 28. 363(18):1704-14. [Medline].
Rao SV, Sherwood MW. Isn't it about time we learned how to use blood transfusion in patients with ischemic heart disease?. J Am Coll Cardiol. 2014 Apr 8. 63(13):1297-9. [Medline]. [Full Text].
94 Stiles S. More evidence that blood transfusions raise thrombosis risk in ACS patients. Medscape [serial online]. Available at http://www.medscape.com/viewarticle/818076. December 19, 2013; Accessed: December 30, 2013.
Jneid H, Addison D, Bhatt DL, et al. 2017 AHA/ACC clinical performance and quality measures for adults with ST-Elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2017 Oct 17. 70(16):2048-90. [Medline]. [Full Text].
95 Carvalho VDCV, Silva LCA, Araujo RM, et al. Acute coronary syndrome: Relationship between genetic variants and TIMI risk. Cytokine. 2018 Oct. 110:344-9. [Medline].
Zocca P, Kok MM, van der Heijden LC, et al. High bleeding risk patients with acute coronary syndromes treated with contemporary drug-eluting stents and clopidogrel or ticagrelor: Insights from CHANGE DAPT. Int J Cardiol. 2018 Oct 1. 268:11-7. [Medline]. [Full Text].
96 Ciliberti G, Coiro S, Tritto I, et al. Predictors of poor clinical outcomes in patients with acute myocardial infarction and non-obstructed coronary arteries (MINOCA). Int J Cardiol. 2018 Sep 15. 267:41-5. [Medline].
97 R, Carasso S, Lavi I, Amir O. The risk for a first acute coronary syndrome in patients treated with different types of antidepressants: a population based nested case-control study. Int J Cardiol. 2018 Sep 15. 267:28-34. [Medline].







































Comentarios